In a new study, researchers from the Will Cornell School of Medicine in the United States have developed an innovative human neuron model that can effectively simulate the diffusion of tau protein aggregates in the brain – a process that is responsible for cognitive decline in Alzheimer’s disease and frontotemporal dementia. Through this new model, they discovered novel therapeutic targets that may prevent tau diffusion. This preclinical study is a significant advancement in Alzheimer’s disease research. The relevant research results were published online in the Cell, under the title “Human iPSC 4R tauopathy model uncovers modifiers of tau propagation”.
Dr. Li Gan, co-author of the paper, said, “Currently, there is no therapy that can prevent the diffusion of tau aggregates in the brains of Alzheimer’s disease patients. Our tau diffusion human neuron model overcomes the limitations of previous models and reveals potential targets previously unknown for drug development.”
Our Featured Products
| Cat.No. | Product Name | Source | Species | Tag |
| TAU-301315H | Recombinant Human TAU protein, GST-tagged | E.coli | Human | GST |
| tau-38D | Recombinant Drosophila melanogaster tau Protein (Full length) | E.coli | Drosophila melanogaster | N/A |
| MAPT-516H | Active Recombinant Human MAPT Protein | E.coli | Human | N/A |
| MAPT-30090TH | Recombinant Human MAPT | E.coli | Human | N/A |
| MAPT-1572H | Recombinant Human Microtubule-Associated Protein Tau | E.coli | Human | N/A |
| MAPT-158H | Recombinant Human Tau-441 (50-441) | E.coli | Human | N/A |
| MAPT-169H | Recombinant Human Tau-441 (151-391) | E.coli | Human | N/A |
| MAPT-168H | Recombinant Human Tau-441 (127-421) | E.coli | Human | N/A |
Human induced pluripotent stem cells (iPSCs) can develop into any cell in the human body and can be induced into neurons in laboratory culture dishes, thereby establishing a brain disease model. However, it is almost impossible to establish a tau propagation model in these formed neurons, as tau propagation requires decades of aging in the brain.
Dr. Gan and his team used CRISPR technology to modify the genome of human iPSCs, promoting their expression of tau forms associated with diseased aging brains. Dr. Gan said, “This model changes the game rules and can simulate the diffusion of tau in neurons within weeks, a process that typically takes decades in the human brain.”
To prevent the spread of tau, the Gan team used CRISPRi screening technology to inactivate one thousand genes to determine their role in tau transmission. They discovered 500 genes that have a significant impact on tau abundance.
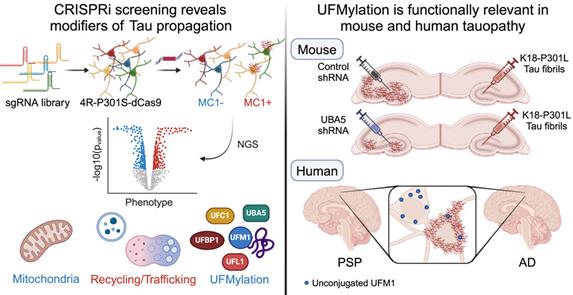
Celeste Parra Bravo, a doctoral student in neuroscience at the Graduate School of Medical Sciences at Weill Cornell Medical School and co-first author of the paper who conducted research at Gan Laboratory said, “CRISPRi technology allows us to use unbiased methods to find drug targets, without being limited to what other scientists have previously reported.”
One of the findings includes the UFMylation cascade, a cellular process involving the connection of a small protein called UFM1 to other proteins. The connection between this process and tau diffusion was previously unknown. Postmortem studies on the brains of Alzheimer’s disease patients have found changes in UFMylation, and these authors have also found in preclinical models that inhibiting the enzymes required for UFMylation can prevent the spread of tau in neurons.
Dr. Shiaoching Gong, co-author of the paper and associate professor of neuroscience at the Apel Institute of Weill Cornell Medical School, said, “Inhibiting UFMylation can prevent the spread of tau in human neurons and mouse models, which is particularly encouraging for us.”
Dr. Gan said that many treatment methods for Alzheimer’s disease initially showed promise in mouse models, but were not successful in clinical trials. With this new human cell model, she is optimistic about the future path. “Our discovery in human neurons has opened the door to the development of new therapies that could truly change the fate of patients with this devastating disease.”
Related Products and Services
Alzheimer’s Disease-related proteins
Protein Expression and Purification Services
Reference
Celeste Parra Bravo et al. Human iPSC 4R tauopathy model uncovers modifiers of tau propagation. Cell, 2024, doi:10.1016/j.cell.2024.03.015.