Transforming Growth Factor beta (TGF-β) Superfamily

🧪 BMP2-01H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 283-396 aa
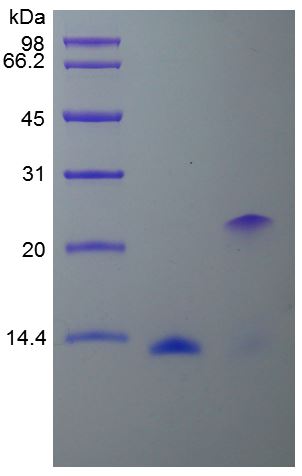
🧪 BMP5-05H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: 324-454 aa

🧪 BMP7-10H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length:
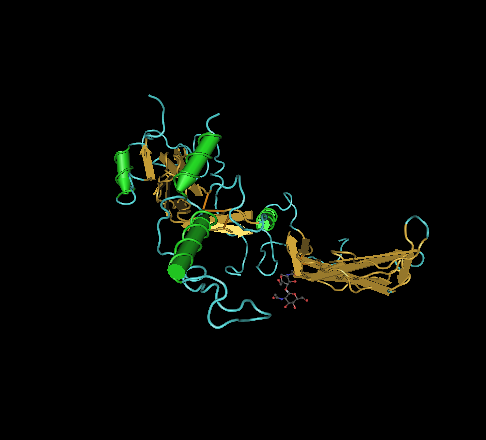

🧪 ACVR2A-195H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: 1-134 aa


🧪 ACVR2B-196H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: 1-134 aa

🧪 ACVR2B-197H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: 1-134 aa

🧪 ACVR2B-198M
Source: HEK293
Species: Mouse
Tag: Fc&His
Conjugation:
Protein Length: Met1-Thr134
🧪 Bmpr1a-247M
Source: HEK293
Species: Mouse
Tag: Fc&His
Conjugation:
Protein Length: Met1-Arg152
🧪 BMP2-16H
Source: HEK293
Species: Human/Mouse/Rat/Rhesus/Canine
Tag: Fc
Conjugation:
Protein Length: Gln283-Arg396
🧪 FSTL1-714H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Met1-Ile308
🧪 Gfra1-722M
Source: HEK293
Species: Mouse
Tag: His
Conjugation:
Protein Length: 1-425 a.a.
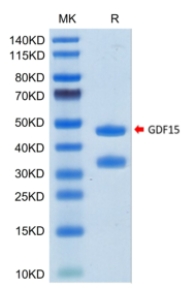
🧪 GDF15-204H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: Ala197-Ile308
🧪 TGFBR2-152H
Source: Human Cells
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: 1-159 a.a.
🧪 FST-611H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 288
🧪 TGFB1-126H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 279-390 a.a.
SubCategories
TGF-β—Product Overview
The transforming growth factor beta (TGF-β) superfamily is a group of multifunctional cytokines that regulate diverse cellular processes, including proliferation, differentiation, apoptosis, and immune responses. This superfamily includes TGF-βs, bone morphogenetic proteins (BMPs), activins, and inhibins, which play critical roles in embryonic development, tissue homeostasis, and disease progression. Due to their involvement in cancer, fibrosis, and autoimmune diseases, the TGF-β superfamily proteins are being widely investigated for their therapeutic potential. Creative BioMart offers a comprehensive selection of recombinant TGF-β superfamily proteins and related products to support research in cell signaling, regenerative medicine, and drug discovery.
Jump to Section
Product Families
-
GDF (Growth Differentiation Factor) Ligands & Receptors
-
ACT (Activin) Family & Receptors & Modulators
-
INHCT (Inhibin) Family
-
BMP Ligands & Receptors
-
GDNF Ligands & Receptors
-
TGF-β Ligands & Receptors
Background
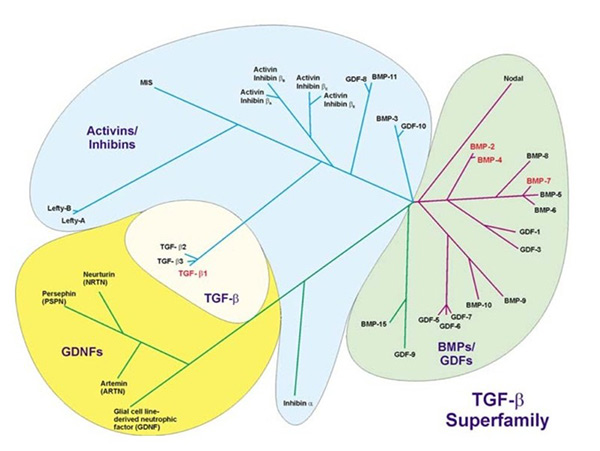
The TGF-β superfamily encompasses over 30 members, each sharing a conserved structure characterized by a cystine knot motif. Notable members include TGF-βs, BMPs, GDFs, activins, and inhibins. These proteins are synthesized as precursor molecules, consisting of a signal peptide, a pro-domain (latency-associated peptide), and a mature peptide. The pro-domain facilitates proper folding and dimerization, while the mature peptide is responsible for receptor binding and signal transduction.
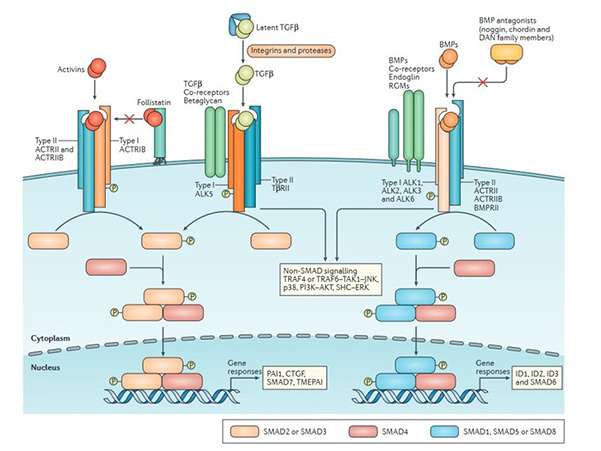
TGF-β superfamily members signal through a heteromeric complex of type I and type II serine/threonine kinase receptors. Upon ligand binding, the type II receptor phosphorylates the type I receptor, which then propagates the signal intracellularly via receptor-regulated SMAD proteins (R-SMADs). These R-SMADs partner with co-SMADs and translocate to the nucleus to regulate target gene expression. Additionally, non-SMAD pathways, such as MAPK, PI3K/AKT, and Rho-like GTPase pathways, can be activated, contributing to the diversity of cellular responses.
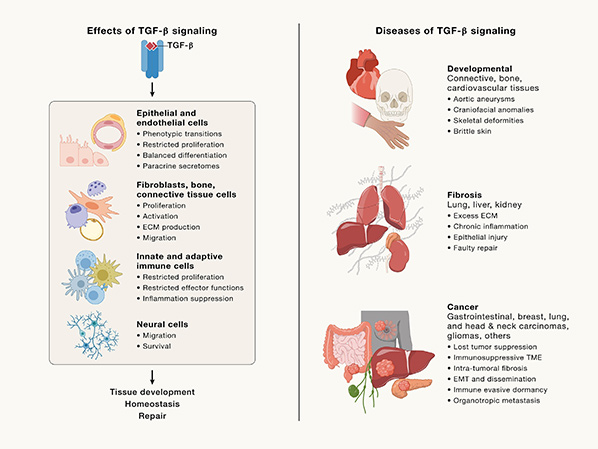
TGF-β superfamily members regulate embryonic development, tissue homeostasis, and disease processes. They influence cell fate, organogenesis, proliferation, differentiation, and apoptosis. BMPs support bone and cartilage formation, while activins and inhibins regulate reproduction. Dysregulation leads to diseases, including cancer, where TGF-β acts as a tumor suppressor early but promotes metastasis later. It also drives fibrosis by inducing excessive extracellular matrix deposition in organs. Additionally, TGF-β plays roles in immune regulation, cardiovascular diseases, and neurodegenerative disorders, making it a key therapeutic target.
Applications
Cell Growth and Differentiation
TGF-β superfamily proteins regulate cell proliferation, differentiation and apoptosis, and play a critical role in embryonic development and tissue homeostasis. They are widely used in stem cell research and regenerative medicine to control cell fate.
Cancer Research and Therapeutics
TGF-β signaling is involved in tumor progression and suppression, making it a key target in cancer research. These proteins are used to study metastasis, epithelial-mesenchymal transition (EMT), and immune evasion mechanisms in various cancers.
Tissue Engineering and Regeneration
Bone morphogenetic proteins (BMPs), part of the TGF-β superfamily, are essential for bone and cartilage formation. They are widely used in orthopedic research, fracture healing, and tissue engineering for regenerative therapies.
Cardiovascular and Fibrosis Studies
TGF-β plays an important role in immune cell regulation, including T-cell differentiation and suppression of inflammatory responses. It is used in autoimmune disease research, transplantation immunology and in the development of therapeutics for inflammatory diseases.
Immunomodulation and Inflammation
TGF-β signaling is central to fibrosis and cardiovascular disease. Researchers are using these proteins to study cardiac remodeling, vascular development and fibrotic disorders with the goal of developing antifibrotic therapies.
Product Features
-
High Purity & Bioactivity: Our TGF-β superfamily proteins are produced using advanced recombinant expression systems, ensuring >95% purity and verified biological activity.
-
Diverse Selection: We offer a comprehensive range of TGF-β isoforms, BMPs, activins, inhibins and other related signaling proteins to support diverse research applications.
-
Validated Functional Assays: Each lot undergoes rigorous quality control, including Smad pathway activation, proliferation, and differentiation assays, to ensure consistent performance.
-
Multiple Expression Systems: Available in mammalian, insect, and E. coli expression systems for proper folding and post-translational modifications.
-
Flexible Formats: Offered in lyophilized or liquid form, with carrier-free and tag-free options to meet diverse experimental needs.
-
Customizable Solutions: Bulk orders and custom formulations are available to meet specific research and industrial needs.
Case Study
Case 1: TGFB2-TGFBR3 in Aortopathy
Tang et al., 2025. TGFBR3 dependent mechanism of TGFB2 in smooth muscle cell differentiation and implications for TGFB2-related aortic aneurysm.
Pathogenic TGFβ variants predispose patients to thoracic aortic aneurysm and dissection (TAAD), particularly in the aortic root. This study investigated the role of TGFB2 in smooth muscle cell (SMC) differentiation using hiPSC-derived SMCs, CRISPR/Cas9, and tissue samples. TGFB2 uniquely relied on TGFBR3 for signaling, with both enriched in the aortic root media. TGFB2 haploinsufficiency impaired second heart field-derived SMC differentiation, and a TGFB2 variant (TGFB2G276R/+) caused mechanical defects that were reversible by TGFB2 supplementation or genetic correction. These findings highlight the distinct role of TGFB2 in aortic root SMCs and suggest that TGFβ isoform redundancy may attenuate the severity of TAAD in TGFB2 variants.
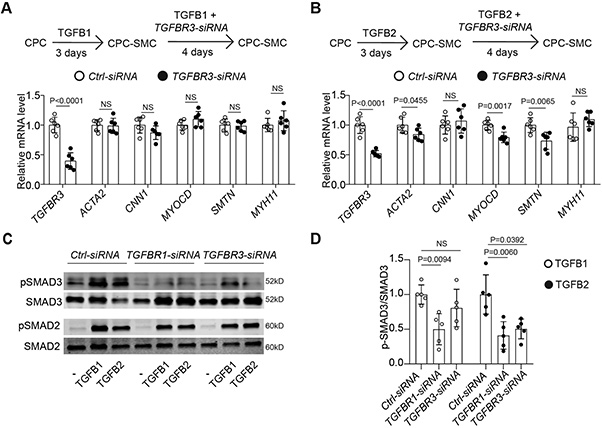
Figure 1. TGFBR3 facilitates TGFB2 signaling transduction during SMC differentiation.
Case 2: BMP7 Modulates Fibrocartilage in OA
Ripmeester et al., 2021. BMP7 reduces the fibrocartilage chondrocyte phenotype.
The fibrocartilage chondrocyte phenotype contributes to the progression of osteoarthritis (OA) by expressing genes associated with adverse outcomes. BMP7, a potential disease-modifying molecule, attenuates chondrocyte hypertrophy and may also have anti-fibrotic effects. This study investigated the role of BMP7 in fibrosis using OA chondrocytes and SW1353 cells. BMP7 decreased fibrosis-related gene expression, collagen type I protein levels, and PAI1, while increasing MMP2 expression and activity. These effects were MMP2 dependent and involved Smad2/3-PAI1 inhibition. The dual effect of BMP7 on hypertrophic and fibrotic chondrocytes suggests its potential as a therapeutic agent for OA by targeting multiple pathological pathways.
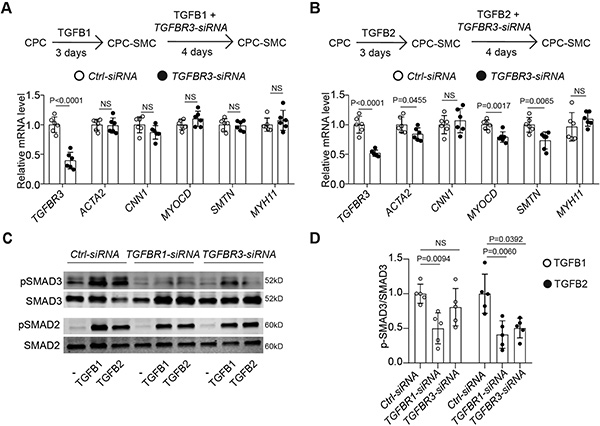
Figure 2. BMP7 increases MMP2 expression and activity in SW1353 cells.
FAQs
-
Q: What types of TGF-β superfamily proteins do you offer?
A: We offer a range of recombinant TGF-β superfamily proteins, including TGF-β isoforms (TGF-β1, TGF-β2, TGF-β3), bone morphogenetic proteins (BMPs), activins, inhibins, and related signaling factors. -
Q: How are your TGF-β superfamily proteins produced?
A: Our proteins are produced using high-quality recombinant expression systems, including E. coli, mammalian cells, and insect cells, to ensure proper folding, bioactivity, and purity.
-
Q: What are the applications of TGF-β superfamily proteins?
A: These proteins are widely used in research on cell signaling, embryonic development, cancer progression, fibrosis, immune regulation, and regenerative medicine.
-
Q: How do you ensure the bioactivity of your TGF-β proteins?
A: Each batch undergoes rigorous quality control including functional assays such as Smad signaling activation, cell proliferation and differentiation studies to confirm biological activity. -
Q: Are your TGF-β proteins available in various formats?
A: Yes, we offer lyophilized and liquid formats as well as carrier-free and tag-free options to meet different experimental needs. -
Q: Can I request bulk orders or custom formulations?
A: Yes, we offer bulk supplies and custom formulations tailored to meet research and industrial needs. Please contact us for further details.

