TGF-β Superfamily Signaling
🧪 KRAS-2569H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-185 aa
🧪 AKT3-131H
Source: Insect Cells
Species: Human
Tag: GST
Conjugation:
Protein Length: 1-479 a.a.
🧪 TGFBR2-152H
Source: Human Cells
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: 1-159 a.a.
🧪 RAF1-1123H
Source: E.coli
Species: Human
Tag: GST
Conjugation:
Protein Length: 50-132 a.a.
🧪 RHOA-1145H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length:
🧪 TGFB1-126H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 279-390 a.a.

🧪 SMURF2-468H
Source: Sf9 Cells
Species: Human
Tag: Flag
Conjugation:
Protein Length: 130-end a.a.
🧪 RAC1-2213H
Source: Insect Cells
Species: Human
Tag: GST
Conjugation:
Protein Length: Met1-Cys189
🧪 TGFBR1-2346H
Source: HEK293
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: Met1-Glu125
🧪 TGFB1-489H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Met 1-Ser 390
🧪 AKT1-786H
Source: Insect Cells
Species: Human
Tag: His
Conjugation:
Protein Length: 1-480 aa
🧪 MAPK8-781H
Source: Insect Cells
Species: Human
Tag: GST
Conjugation:
Protein Length: Met1-Arg427
🧪 PDK1-904H
Source: Insect Cells
Species: Human
Tag: His
Conjugation:
Protein Length: 29-436 a.a.
🧪 RHOA-3975H
Source: Insect Cells
Species: Human
Tag: His
Conjugation:
Protein Length: 1-193 a.a.
🧪 TGFB1-576M
Source: HEK293
Species: Mouse/Rat
Tag: Non
Conjugation:
Protein Length: Ala279-Ser390
Background of TGF-β Signal Pathway
What is TGF-β Signal Pathway?
The pathway is initiated when TGF-β ligands, a family of peptide growth factors, bind to specific cell surface receptors. TGF-β receptors are typically composed of two types: Type I (also known as ALK5) and Type II receptors. The binding of TGF-β to the Type II receptor promotes its interaction with and subsequent activation of the Type I receptor.
Smad-Dependent Signaling: Activation of the Type I receptor leads to phosphorylation of receptor-regulated Smads (R-Smads), which include Smad2 and Smad3. Once phosphorylated, these R-Smads form a complex with the common mediator Smad (Co-Smad), Smad4. The Smad complex then translocates into the nucleus where it acts as a transcription factor to regulate the expression of target genes involved in various cellular responses.
Smad-Independent Signaling: In addition to the Smad-dependent pathway, TGF-β can also activate Smad-independent pathways, such as the MAPK (Mitogen-Activated Protein Kinase) and PI3K/AKT (Phosphatidylinositol 3-Kinase/Protein Kinase B) pathways, which can influence cell behavior.
Depending on the cell type and context, TGF-β signaling can lead to different outcomes, such as promoting cell proliferation, inhibiting cell growth, stimulating cell differentiation, or inducing cell death.
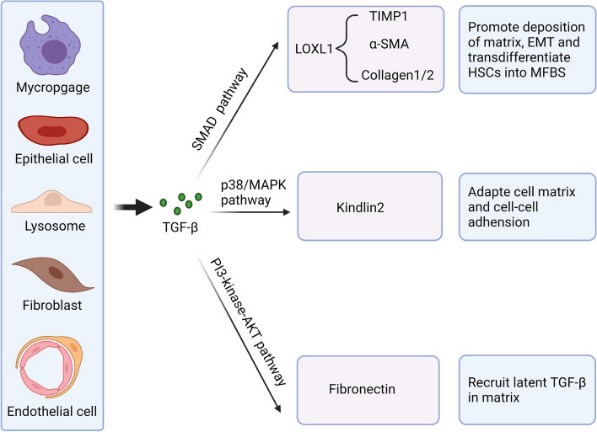
Fig1. Essential functions of TGF-β in fibrosis under pathological conditions. (Dandan Peng, 2022)
Core Components of TGF-β Signal Pathway
TGF-β Ligands
A family of secreted growth factors that include TGF-β itself, as well as related proteins like Activins and Bone Morphogenetic Proteins (BMPs). TGF-β is a highly conserved secreted protein that is present in a variety of cell types. It exists in an inactive form in the extracellular matrix and can be activated by specific enzyme digestion or other signaling events. Activins are members of the TGF-β superfamily and regulate cell differentiation and proliferation primarily by interacting with Smad proteins. BMPs are another important member of the TGF-β superfamily, which plays an important role in bone and cartilage formation, neurodevelopment, and organogenesis.
TGF-β Receptors
Type II Receptors: These are the first receptors to bind the TGF-β ligand and are essential for signaling. They possess a kinase domain that phosphorylates the Type I receptor.
Type I Receptors (also known as ALK5 for TGF-β): Once the Type II receptor is activated, it recruits and phosphorylates the Type I receptor, which is crucial for downstream signaling.
Smad Proteins
Receptor-Regulated Smads (R-Smads): For TGF-β, these are Smad2 and Smad3. They are phosphorylated by the activated Type I receptor.
Common-Mediator Smad (Co-Smad): Smad4 acts as a common partner for R-Smads to form a complex that translocates to the nucleus.
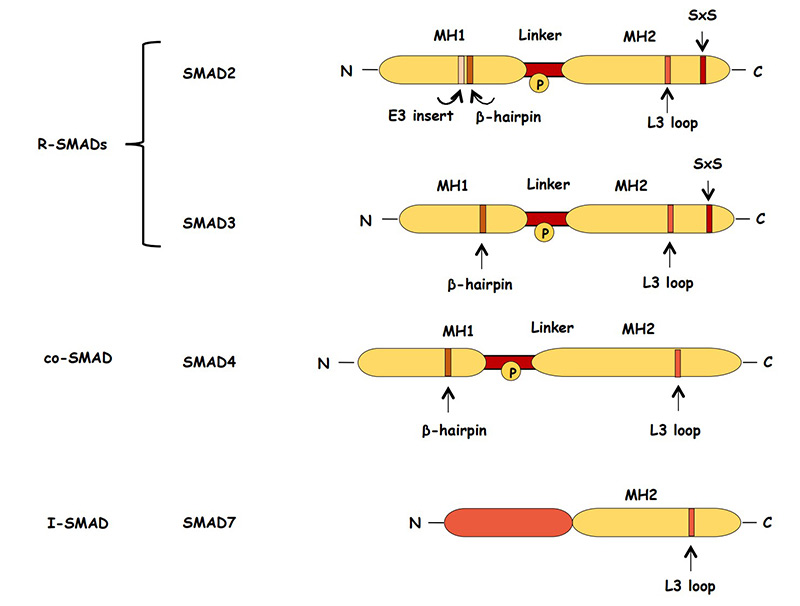
Fig2. Schematic representation of structure of the SMAD proteins. (Kalliopi Tzavlaki, 2020)
TGF-β Signal Pathway Related Diseases
The TGF-β signal pathway is intricately involved in the regulation of cellular processes such as proliferation, differentiation, migration, and apoptosis. Dysregulation of this pathway has been associated with a variety of diseases, including:
TGF-β signaling plays a dual role in cancer. It acts as a tumor suppressor in the early stages by inhibiting cell proliferation, but it can also promote tumor progression, metastasis, and angiogenesis in later stages by inducing epithelial-to-mesenchymal transition (EMT) and immunosuppression.
Abnormal TGF-β signaling can lead to the accumulation of extracellular matrix (ECM) proteins, resulting in tissue fibrosis. This is observed in conditions like pulmonary fibrosis, liver cirrhosis, and kidney fibrosis.
TGF-β is a potent immunosuppressive cytokine, and alterations in its signaling can contribute to the development of autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and multiple sclerosis (MS).
On the whole, TGF-β signal pathway is essential for embryonic development, tissue repair, and immune regulation. Dysregulation of TGF-β signaling is associated with several pathologies, including fibrosis, autoimmune diseases, and cancer, where it can act as both a tumor suppressor and a promoter of tumor progression.
Therapeutic Potential
In the early stages of cancer, TGF-β often has a tumor suppressor effect, but in advanced stages, it can promote tumor invasion, metastasis, and immune escape. Therefore, developing drugs to regulate the TGF-β signaling pathway may help treat certain types of cancer. TGF-β plays a role in vascular development and proliferation of vascular smooth muscle cells, and regulating TGF-β signaling may help treat atherosclerosis, hypertension, and other cardiovascular diseases. The TGF-β signaling pathway plays a role in neurodevelopment and neural repair and may be a potential target for the treatment of Alzheimer's disease and other neurodegenerative diseases.
Due to TGF-β's role in cell proliferation and differentiation, modulating its signaling pathway may help improve wound-healing processes and tissue engineering strategies. TGF-β affects the balance of bone formation and absorption, and the regulation of its signaling pathway may have a positive effect on the treatment of osteoporosis. The regulation of the TGF-β signaling pathway may be used in combination with other therapies (e.g., chemotherapy, radiotherapy, immunotherapy) to improve therapeutic efficacy.
Case Study
Case Study 1: Recombinant Human TGFB1 (TGFB1-120H)
Idiopathic pulmonary fibrosis (IPF) can severely damage lung function, which may result in death. Emodin is a major ingredient of rhubarb and has been proven to protect against lung disruptions. This study focused on the potential medicinal effect of emodin against IPF. The experiment subjects were fully-grown male Sprague-Dawley rats with average weight of 180–220 kg. Histological analyses, Western blotting analysis, quantitative real-time PCR, and statistical analysis were used in the study. Recombinant TGF-β1 in volume of 10 ng/mL was collectively administrated to the rat primary AECs and the A549 cell line for 2 days. The results showed that emodin significantly reduced lung structural distortion, collagen overproduction, massive inflammatory cells infiltration, proinflammatory cytokines expansion, and injuries caused by administration of bleomycin (BLM). Additionally, emodin suppressed the accumulation of p-IκBα and NF-κB, while stimulating the Nrf2-antioxidant signaling process in damaged lungs. Emodin inhibited epithelial-mesenchymal transition (EMT) induced by BLM in the lungs. Moreover, emodin suppressed the TGF-β1 expression and the downstream signal molecules p-Smad-2 and p-Smad-3, which are reinforced by BLM. Emodin can also reverse EMT-like shifts induced by recombinant TGF-β1 in alveolar epithelial cultured cells.
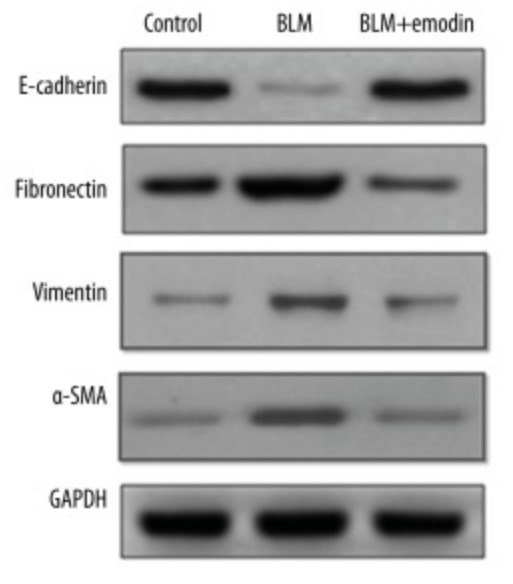
Fig1. Expression levels of E-cadherin, fibronectin vimentin, and α-SMA were measured by Western blot assay. (Sheng-Lan Tian, 2018)
Case Study 2: Recombinant Human TGFBR1 (TGFBR1-1495H)
In ovarian cancer cells, galloylated catechins were recently demonstrated to target the transforming growth factor (TGF)-β-mediated control of the epithelial-mesenchymal transition process. The specific impact of the galloyl moiety on such signaling, however, remains poorly understood. Here, the researchers questioned whether the sole galloyl moiety interacted with TGF-β-receptors to alter signal transduction and chemotactic migratory response in an ES-2 serous carcinoma-derived ovarian cancer cell model. In line with the LogP and LogS values of the tested molecules, we found that TGF-β-induced Smad-3 phosphorylation and cell migration were optimally inhibited, provided that the lateral aliphatic chain of the galloyl moiety reached 8–10 carbons. Functional inhibition of the TGF-β receptor (TGF-βR1) kinase activity was supported by surface plasmon resonance assays showing direct physical interaction between TGF-βR1 and the galloyl moiety. In silico molecular docking analysis predicted a model where galloylated catechins may bind TGF-βR1 within its adenosine triphosphate binding cleft in a site analogous to that of Galunisertib, a selective adenosine triphosphate-mimetic competitive inhibitor of TGF-βR1.
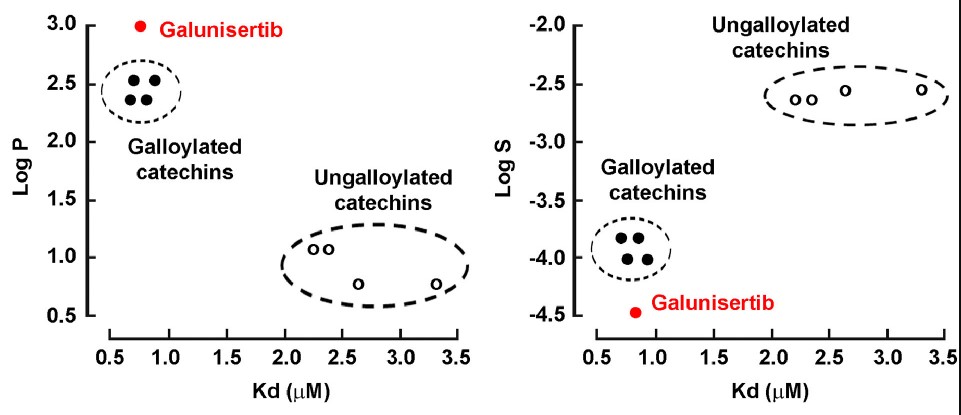
Fig2. Dissociation constants for the binding of galloylated catechins to the TGF-βR1 kinase domain. (Audrey-Ann Sicard, 2021)
Case Study 3: Bovine Transforming Growth Factor beta-1 Reference standard (TGFB1-96B)
Transforming growth factor-β1 (TGF-β1) plays a crucial role in chronic inflammation in various tissues, and is related to inflammation-caused organ fibrogenesis associated with the epithelial-mesenchymal transition (EMT) and the deposition of the extracellular matrix (ECM). However, the effect of TGF-β1 on bovine mammary epithelial cells (BMECs) with mastitis, and its mechanism, remain unknown. The researchers analyzed the level of TGF-β1 in inflamed mammary tissues and cells using western blotting. BMECs were treated with TGF-β1, and EMT-related gene and protein expression changes were evaluated using quantitative real-time polymerase chain reaction (qPCR), western blotting, and immunofluorescence. We also inhibited the TGF/Smad signaling pathway using a receptor inhibitor, and analyzed EMT-related protein expression by western blotting. The results showed the TGF-β1 level was up-regulated in mammary tissues with mastitis and in inducible inflammatory BMECs. TGF-β1 treatment activated the TGF/ Smad signaling pathway in BMECs during their transition to the EMT phenotype, as indicated by morphological changes from a cobblestone-like shape to a spindle-like one. TGF-β1 treatment also up-regulated the expression of α-smooth muscle actin, vimentin, and collagen I, albumin, and down-regulated the expression of E-cadherin both in mRNA level and protein level.
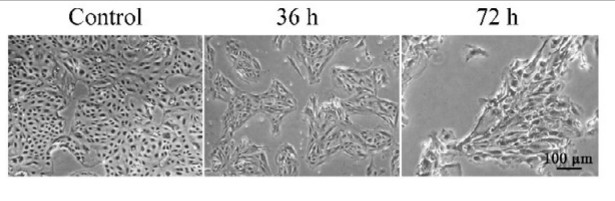
Fig3. BMECs showing obvious morphology changes during TGF-β1 treatment under a phase-contrast microscope. (Qing Chen, 2017)
Related Products
The TGF-β Signal Pathway is a complex and multifaceted system that plays a critical role in regulating a wide array of cellular processes, including cell proliferation, differentiation, migration, and apoptosis. The TGF-β superfamily includes TGF-βs, Activins, Nodal, and Bone Morphogenetic Proteins (BMPs), among others. Creative BioMart can provide a list of core protein products of the TGF-β Signal Pathway and the TGF-β superfamily to help you with your researches. Please feel free to contact us if you’re interested.


