Uncategorized Sunday, 2025/07/13
This study adds important new details to a cell protein network that is difficult to characterize. Dr. Salgia and his team focused on paxillin, a protein that allows cells to adapt based on environmental changes. This helps cancer cells evolve and evade detection while triggering resistance to treatment.
In a new study, an international research team led by scientists from City of Hope provided the most comprehensive elucidation to date of an elusive target in cancer treatment. They highlighted a complex signaling process involving paxillin that may be sensitive to treatment, even though the protein is in a flux state. Paxillin is a focal adhesion protein that acts as a hub for connecting other proteins. The related research findings were published in the journal Science Advances.
Dr. Ravi Salgia, co-corresponding author of the paper and professor of medical oncology at City of Hope Comprehensive Cancer Center, said, "Interfering with the interaction of paxillin and focal adhesions has direct implications for cancer treatment. This could lead to precision therapies targeting specific paxillin functions that are dominant in cancer cells but uncommon in healthy cells."
Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| FAK-12647H | Recombinant Human FAK, His-tagged | E.coli | Human | His | C-term-298a.a. | |
| FAK-301450H | Recombinant Human FAK protein, GST-tagged | E.coli | Human | GST | Ile381-Arg678 |
|
| PTK2-1272C | Recombinant Chicken PTK2 Protein (376-683 aa), GST-tagged | E.coli | Chicken | GST | 376-683 aa |
|
| PTK2-6827H | Recombinant Human PTK2 protein, His & T7-tagged | E.coli | Human | His&T7 | Gly798~Ala1041 |
|
| PXN-2083H | Recombinant Human PXN, His-tagged | E.coli | Human | His | 1-267aa | |
| PXN-029H | Recombinant Human PXN Protein, Trx, His and S-tags-tagged | E.coli | Human | His&S&Trx |
|
This study adds significant new details to a cell protein network that is hard to characterize. Dr. Salgia and his team focused on paxillin, a protein that allows cells to adapt to environmental changes. This helps cancer cells evolve and evade detection while contributing to treatment resistance. Dr. Salgia’s team has been committed to elucidating the function of paxillin for over thirty years. He and his colleagues first cloned the full-length sequence of the human paxillin coding gene at Harvard University in 1995.
To better understand the role of paxillin, Dr. Salgia's team turned to its primary binding partner, focal adhesion kinase (FAK). This research process was challenging. These two proteins share a large number of residues for binding and are in a state of continuous dynamic change. Paxillin itself is also a highly disordered protein.
Dr. Salgia's team narrowed their research to characterize only the most relevant structures. Ultimately, they discovered a stable structure in stark contrast to the disordered state. When paxillin interacts with the C-terminal targeting domain of FAK at specific docking sites, they must compress and maintain that state to fit the limited space. However, they still maintain high flexibility in interacting with the broader focal adhesion network.
"Our findings reveal a new mechanism of protein interaction rarely studied in the literature, suggesting that such mechanisms may apply to other disordered proteins," Dr. Salgia stated. "This study has broad implications for disordered proteins in general."
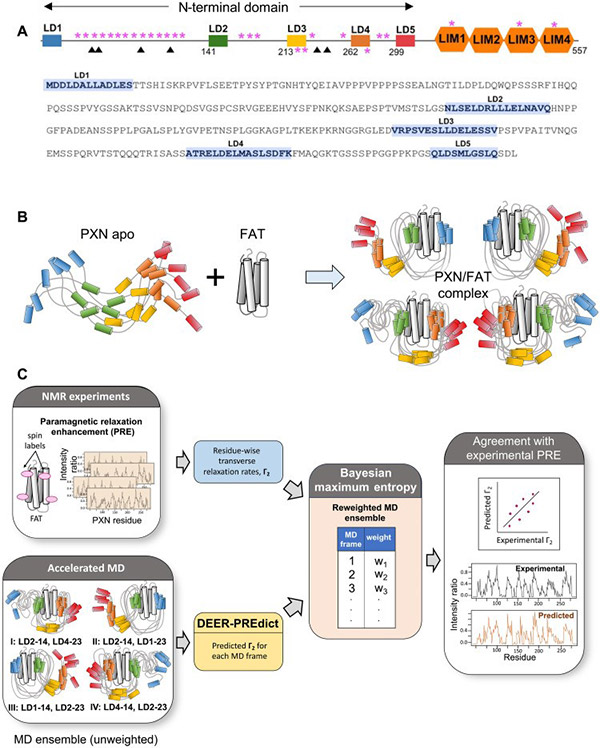
Fig1. Deriving the conformational ensemble of human PXN bound to FAT
Such protein interactions are often considered difficult to control therapeutically due to a lack of clear drug-targeting sites. But by capturing the observed phenomena, Dr. Salgia's team constructed a model that may help other researchers identify dynamic targets.
This finding was the result of much ingenious laboratory work. The team utilized a spectroscopic technique related to medical magnetic resonance imaging (MRI) to better understand the structural characteristics of paxillin. They then combined spectroscopy with dynamic simulation to show how paxillin binds to the C-terminal targeting domain of FAK. Finally, they created a computer 3D model to demonstrate the specific process of this interaction.
Dr. Supriyo Bhattacharya, co-corresponding author of the paper and assistant research professor in the Department of Computational and Quantitative Medicine at City of Hope, said, "The combination of all these methods allowed us to characterize the structural features of the paxillin-FAK interaction more accurately than any single method alone."
Related Products & Services
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Immune Checkpoint Proteins
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Supriyo Bhattacharya et al, Conformational dynamics and multimodal interaction of Paxillin with the focal adhesion targeting domain, Science Advances (2025). DOI: 10.1126/sciadv.adt9936.
