Uncategorized Sunday, 2025/07/13
CAR-T (Chimeric Antigen Receptor T-Cell Immunotherapy) is a novel cell-based therapy that has been around for several years. It has only recently been refined and applied in clinical settings. This therapy has shown remarkable efficacy in treating acute leukemia and non-Hodgkin lymphoma and is considered one of the most promising cancer treatment approaches. Like all technologies, CAR-T has undergone a long evolutionary process, during which it has gradually matured.
The crux of this new therapeutic strategy lies in an artificial receptor called the chimeric antigen receptor (CAR), which can identify target cells. After genetic modification, patients' T cells can express this CAR. In clinical trials, scientists extract some T cells from patients through a process similar to dialysis. They then genetically modify these cells in the laboratory by introducing the gene encoding the CAR, enabling the T cells to express this new receptor. After proliferating the genetically modified T cells in the lab, they are infused back into the patient's body. These T cells use their expressed CAR receptors to bind to molecules on the target cell surface, triggering an internal signal that so strongly activates the T cells that they rapidly destroy the target cells.
In recent years, in addition to treating acute leukemia and non-Hodgkin lymphoma, CAR-T immunotherapy has been improved and applied to solid tumors, autoimmune diseases, HIV infections, and cardiovascular diseases, showing broader application prospects. Based on this, the editor has summarized the latest advances in CAR-T cell therapy.
Groundbreaking Cell Study: CAR-T Cells Expressing Natural TCR Can Specifically Target Cancer Cells Without Affecting Healthy Tissues
DOI: 10.1016/j.cell.2025.03.017
A scientific relay spanning two decades in the field of cancer therapy is brewing a revolutionary breakthrough. In a new study, researchers from the University of Montreal, the National Cancer Institute of the United States, and other institutions have successfully created more effective immune cells capable of fighting cancer without damaging healthy tissues. The findings were published in the journal Cell.
The research team integrated the high-throughput robotic platform of the Altan-Bonnet Laboratory (capable of analyzing over 10,000 T cell-target cell interaction events per day) with the multiscale mathematical models of Professor François' team. For the first time, they revealed the mystery of the bidirectional regulation of the T cell receptor (TCR): "The TCR is not only the accelerator of the immune response but can also transform into a precise braking system."
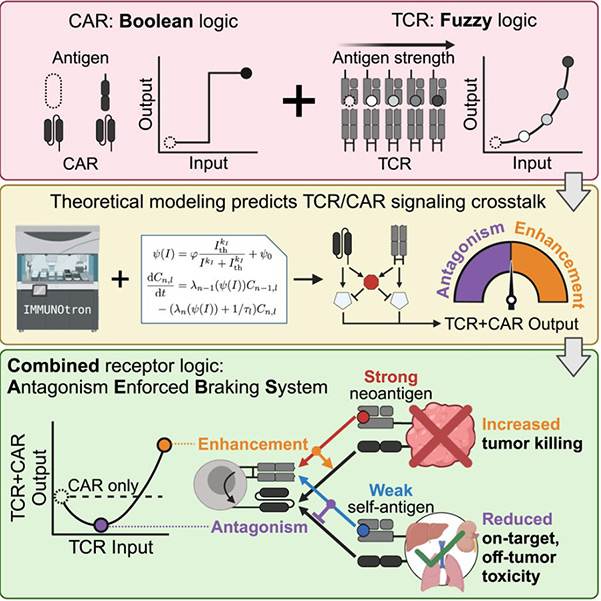
Fig1. Engineering TCR-controlled fuzzy logic into CAR T cells enhances therapeutic specificity graphical abstract
The research team fully utilized the naturally occurring receptor in T cells—the T cell receptor (TCR), which can distinguish healthy cells from cancer cells by recognizing different cell surface proteins. However, previous studies have shown that TCR is not very effective in combating tumors. Despite this, the research team successfully combined these two strategies and designed CAR-T cells containing TCR. Through mathematical modeling, they found that when TCR bindsly weak to surface proteins on healthy cells, it activates inhibitory signals that counteract CAR's attack commands. In contrast, strong binding to cancer cells enhances killing power. Based on this principle, the team developed the "Antagonist-Enforced Braking System" (AEBS), enabling immune cells to precisely switch between "combat mode" and "protection mode" in the tumor microenvironment.
In a humanized solid tumor mouse model, the novel dual TCR/CAR T cells demonstrated remarkable performance. These dual TCR/CAR T cells enhanced anticancer efficacy by 50% compared to traditional CAR T cells while reducing toxicity to healthy tissues by 90%! "For the first time, we have shown that leveraging the natural inhibitory dialogue between receptors allows the immune system to precisely excise tumors like a surgeon," the research team excitedly pointed out.
Ultrasound "Remote Control" of CAR-T Cells! Cell: A New Technology Called EchoBack-CAR T Cells Has the Potential to Be a "Game Changer" in Solid Tumor Therapy
DOI: 10.1016/j.cell.2025.02.035
On the battlefield of cancer therapy, immune cells are like the body's "special forces," and CAR-T cell therapy has been a highly acclaimed "sharp weapon" in recent years. However, when facing elusive solid tumors, traditional CAR-T cell therapy often falls short, much like warriors encountering a solid fortress that is difficult to breach. Recently, researchers from the University of Southern California published a research report titled "Engineering Sonogenetic EchoBack-CAR T Cells" in the journal Cell, introducing a new technology called EchoBack-CAR T cells, which promises to be a "game changer" in solid tumor therapy.
In this study, researchers successfully developed EchoBack-CAR T cells through innovative design, bringing new hope to solid tumor treatment. The core advantage of EchoBack-CAR T cells lies in their unique design and superior performance.
Researchers identified an ultra-sensitive heat shock promoter and integrated it with the positive feedback loop of CAR signaling, enabling EchoBack-CAR T cells to achieve sustained CAR expression under focused ultrasound (FUS) stimulation. Taking EchoBack-hGD2CAR T cells targeting ganglioside GD2 as an example, they demonstrated robust cytotoxicity and persistence in a 3D glioblastoma (GBM) model. In mouse experiments, EchoBack-hGD2CAR T cells effectively inhibited GBM growth, exhibited no off-target toxicity, and outperformed traditional CAR T cells.
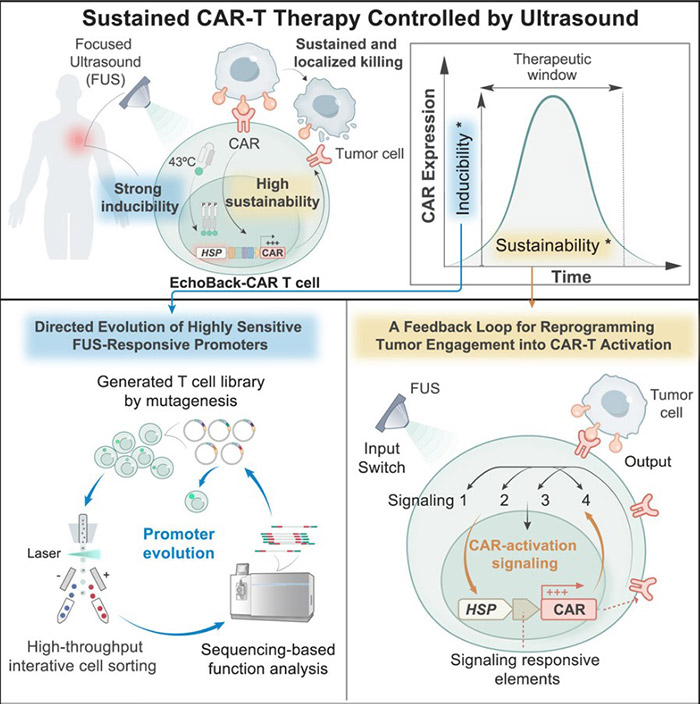
Fig2. Engineering sonogenetic EchoBack-CAR T cells graphical abstract
Blood: New Study Suggests CAR-T Cells Secreting TIM-3 Decoys May More Effectively Treat B-ALL Leukemia
DOI: 10.1182/blood.2024025440
B-cell acute lymphoblastic leukemia (B-ALL) is a life-threatening and highly aggressive cancer. It is the most common childhood cancer, accounting for 35% of pediatric cancer cases, but it can affect individuals of any age. While CAR-T cell therapy has significantly improved the prognosis of B-ALL patients, over 50% of cases still relapse, leaving many patients with limited treatment options. Ongoing research aims to address this challenge and enhance the efficacy of CAR-T therapy.
One of the main challenges in CAR-T cell therapy for B-ALL is relapse. A key question researchers are investigating is why CAR-T cells, as a living cell therapy, sometimes fail to respond to cancer cells even when they are present. In a new study, researchers explored the interactions between tumor cells and immune cells, including CAR-T cells, and identified a promising clue. The findings were published in the journal Blood.
The study confirmed that B-ALL may exploit the body's natural defense mechanisms, particularly immune checkpoint pathways. These pathways typically serve as "off switches" that signal immune cells to cease attack after an infection or threat has been eliminated. However, B-ALL can hijack this system to evade immune system attacks.
Fig3
In the case of B-ALL, researchers found that relapsed leukemia cells exhibit abnormally high levels of galectin-9, a component of the immune checkpoint system. On the other hand, CAR-T cells express high levels of TIM-3, a receptor that usually interacts with galectin-9 to suppress immune activity. B-ALL appears to utilize this interaction to "shut down" CAR-T cells, allowing the cancer to persist.
Related Proteins
To address this issue, researchers created a TIM-3 decoy, a soluble version of the TIM-3 protein that can block this ligand-receptor interaction. The idea is that the decoy can prevent B-ALL from deactivating CAR-T cells without directly signaling them to stop immune activity.
In laboratory tests using transgenic mice with human B-ALL cells, their findings were promising. CAR-T cells genetically modified to secrete the TIM-3 decoy demonstrated enhanced antileukemia effects and long-term persistence.
Nat Med: New Study Identifies a Rare Side Effect of CAR-T Cell Therapy for Blood Cancers—T Cell Lymphoma
DOI: 10.1038/s41591-025-03499-9
Researchers from the University Medical Center Leipzig, the Fraunhofer Institute for Cell Therapy and Immunology, and the University Hospital Cologne identified and analyzed a rare but severe side effect of an innovative blood cancer therapy in a study published in Nature Medicine titled "Multiomic profiling of T cell lymphoma after therapy with anti-BCMA CAR T cells and GPRC5D-directed bispecific antibody."
A 63-year-old multiple myeloma patient developed T cell lymphoma in the blood, skin, and gut nine months after receiving CAR-T cell therapy at the University Hospital Cologne. This tumor arose from the genetically modified T cells used in the treatment.
Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| TNFRSF17-552H |
Active Recombinant Human TNFRSF17, Fc-tagged, Biotinylated

|
Human Cells | Human | Fc | 2-54 a.a. | |
| TNFRSF17-122H |
Active Recombinant Human TNFRSF17 Protein, His-tagged
|
HEK293 | Human | His | Met1-Ala54 | |
| TNFRSF17-1817H |
Recombinant Human TNFRSF17 protein, hFc-tagged
|
HEK293 | Human | Fc | Met1-Ala54 | |
| TNFRSF17-1827C |
Active Recombinant Cynomolgus TNFRSF17 protein, Fc & Avi-tagged, Biotinylated
|
HEK293 | Cynomolgus | Avi&Fc | AA Met 1 - Ala 53 | |
| TNFRSF17-2348H |
Active Recombinant Human TNFRSF17(Met 1- Ala 54), Fc/His tagged
|
HEK293 | Human | Fc&His | Met 1- Ala 54 |
Professor Maximilian Merz, a co-corresponding author of the paper, stated, "This is one of the first documented cases of such lymphoma following CAR-T cell therapy. The results of this study will help us better understand the risks associated with this treatment and may prevent these risks in the future."
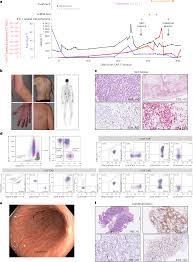
Fig4. Persistent CAR T cell proliferation with cutaneous and gastrointestinal manifestations after cilta-cel therapy in a patient with MM.
Related Products & Services
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Immune Checkpoint Proteins
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Engineering TCR-controlled fuzzy logic into CAR T cells enhances therapeutic specificity. Kondo, Taisuke et al. Cell, Volume 188, Issue 9, 2372 - 2389.e35
- Engineering sonogenetic EchoBack-CAR T cells. Liu, Longwei et al. Cell, Volume 188, Issue 10, 2621 - 2636.e20
- Aïda Falgàs, Rodrigo Lázaro-Gorines, Samanta Romina Zanetti, et al.; A TIM-3–Fc decoy secreted by engineered T cells improves CD19 CAR T-cell therapy in B-cell acute lymphoblastic leukemia. Blood 2025; 145 (22): 2599–2613. doi: https://doi.org/10.1182/blood.2024025440
- Braun, T., Rade, M., Merz, M., Klepzig, H., Große, F., Fandrei, D., Pham, N., Kreuz, M., Kuhn, C. K., Kuschel, F., Löffler, D., et al. (2025). Multiomic profiling of T cell lymphoma after therapy with anti-BCMA CAR T cells and GPRC5D-directed bispecific antibody. Nature Medicine, 31(4), 1145-1153. https://doi.org/10.1038/s41591-025-03499-9
- Perica K, Kotchetkov IS, Mansilla-Soto J, et al. HIV immune evasin Nef enhances allogeneic CAR T cell potency. Nature. 2025 Apr;640(8059):793-801. doi: 10.1038/s41586-025-08657-0. Epub 2025 Jan 30. PMID: 39884316.
- Adachi, Y., Terakura, S., Osaki, M., et al. (2024). Cullin-5 deficiency promotes chimeric antigen receptor T cell effector functions potentially via the modulation of JAK/STAT signaling pathway. Nature Communications, 15(1), 1-14. https://doi.org/10.1038/s41467-024-54794-x
- Cochrane RW, Robino RA, Granger B, et al. High-affinity chimeric antigen receptor signaling induces an inflammatory program in human regulatory T cells. Mol Ther Methods Clin Dev. 2024 Nov 18;32(4):101385. doi: 10.1016/j.omtm.2024.101385. PMID: 39687729; PMCID: PMC11647616.
- Hosoya E, Ando J, Kinoshita S, Furukawa Y, Toyoshima Y, Azusawa Y, Mitsumori T, Sato E, Takano H, Tsukune Y, Watanabe N, Takaku T, Yasuda H, Hamano Y, Sasaki M, Nojiri S, Ishii M, Ando M. Eleven cases of laryngeal edema after tisagenlecleucel infusion: a 3-year single center retrospective study of CD19-directed chimeric antigen receptor T-cell therapy for relapsed and refractory B-cell lymphomas. Haematologica. 2025 Mar 1;110(3):777-783. doi: 10.3324/haematol.2024.286169. PMID: 39415689; PMCID: PMC11873693.
- Westhaus A, Barba-Sarasua E, Chen Y, et al. Tailoring capsid-directed evolution technology for improved AAV-mediated CAR-T generation. Mol Ther. 2025 Jun 4;33(6):2801-2818. doi: 10.1016/j.ymthe.2024.12.012. Epub 2024 Dec 12. PMID: 39673125; PMCID: PMC12172172.
- Song F, Tsahouridis O, Stucchi S, et al. A multi-kinase inhibitor screen identifies inhibitors preserving stem-cell-like chimeric antigen receptor T cells. Nat Immunol. 2025 Feb;26(2):279-293. doi: 10.1038/s41590-024-02042-1. Epub 2025 Jan 8. PMID: 39779871; PMCID: PMC11785528.
