PROTAC Targets

🧪 EGFR-30H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: 1-645 aa
🧪 FLT4-37H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Met1-Ile776

🧪 AKT3-131H
Source: Insect Cells
Species: Human
Tag: GST
Conjugation:
Protein Length: 1-479 a.a.
🧪 Bmpr1a-247M
Source: HEK293
Species: Mouse
Tag: Fc&His
Conjugation:
Protein Length: Met1-Arg152
🧪 Axl-132M
Source: HEK293
Species: Mouse
Tag: Fc&His
Conjugation:
Protein Length: Met1-Pro443
🧪 BMPR2-133H
Source: HEK293
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: Met1-Ile151
🧪 BMPR2-134H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Met 1-Ile 151
🧪 FGFR1-138H
Source: Human Cells
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: 1-285 a.a.

🧪 EGFR-692H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: 1-640 a.a.
🧪 EPHB2-693H
Source: HEK293
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: Met 1-Leu 543
🧪 EPHB4-696H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Glu17-Ala539

🧪 EPHB4-697H
Source: HEK293
Species: Human
Tag: Non
Conjugation:
Protein Length: 1-539 a.a.
🧪 EPHB6-698H
Source: HEK293
Species: Human
Tag: Non
Conjugation:
Protein Length: 1-579 a.a.
🧪 FGFR2-705H
Source: HEK293
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: Met1-Glu377
🧪 FGFR4-708H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: Met1-Asp369
Can’t find what you are looking for exactly? We can still help! Creative BioMart has focused on protein production and purification for years, and our custom-service team is capable of creating the protein that suits your experimental needs.
What else? We can also provide PROTACs services, including:
- E3 ligase and target protein development service
- Ligand discovery and design service
- PROTAC in vitro evaluation
- PROTAC in vivo evaluation
- Targeted protein degradation service
Backgound
What is PROTAC?
PROTAC (PROteolysis TArgeting Chimeras) is a technology used in drug development that utilizes the body's own natural cell machinery to degrade target proteins. This differs from traditional drugs that either inhibit or activate proteins. PROTACs allow for the removal of specific disease-causing proteins, providing a therapeutic effect. This approach has been used to target proteins involved in various diseases, including cancer and neurodegenerative disorders.
What are the PROTAC target proteins?
PROTAC has the ability to target a wide range of proteins. Some of the target proteins include BRD4, ER, BTK, and FKBP12. However, it's important to note that the list of target proteins can greatly vary depending on the specific PROTAC molecule being used, as these molecules are designed to target specific proteins for degradation.
Targeted protein degraders (TPD) are a new class of therapeutic drugs, which may solve the problem of drug targeting in a new way. Targeted protein degradation agents - including molecular gels and heterobifunctional molecules - are a rapidly developing class of drugs with potential for cancer and other diseases.
Protein degradation targeted chimeric Technology (PROTAC) uses heterobifunctional small molecular compounds to bring the target protein closer to the E3 ligase in cells, and specifically degrades the target protein by using the ubiquitin proteasome protein degradation pathway in vivo. PROTAC molecule is composed of three parts, one end is a specific E3 ligase ligand, the other end is a specific ligand of the target protein, and the middle linker, so as to form a "target protein protac-e3 ligase" ternary complex.
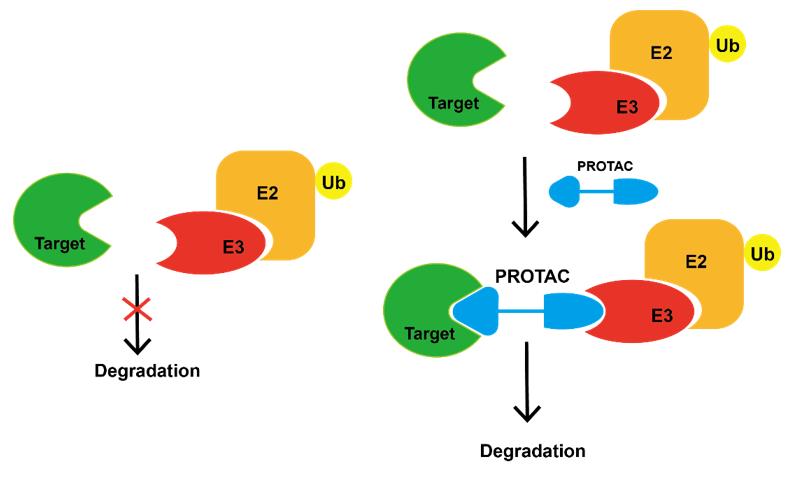
At present, a variety of strategies have been developed for cancer treatment, such as small molecule inhibitors, monoclonal antibodies, RNA interference and CRISPR/Cas9; Due to its unique chemical and biological characteristics, PROTAC has unique advantages and disadvantages in cancer treatment. The advantages of PROTAC include: event driven activity, targeting non pharmaceutical proteins, overcoming traditional drug resistance, etc. Disadvantages include membrane permeability, off target toxicity, etc.
From the perspective of targets, according to the latest statistics in December 2021, more than 130 PROTAC targets have been reported, and the number of degradable targets reported from 2020 to 2021 (about 90) has completely exceeded the total amount of the previous 18 years, indicating that the era of protein degradation is coming.
In the reported studies, researchers prefer to choose kinase as the target of protein degradation. According to incomplete statistics, about 54 kinases can be degraded by protein degrading agents based on PROTAC. At present, 518 kinases have been found. According to the classification of human kinase map, Creative BioMart provides the kinases that can be degraded as "degradable kinases" below. In the "degradable" kinase table, according to the classification method of kinase map, only CK1 and CaMK kinase groups have not reported related PROTAC. RTKs and CMGCS kinases have been reported to have the most degradable targets, 19 and 14, respectively, accounting for more than half of the total number of degradable kinases.
Besides the PROTAC targets, E3 ligases is also important for PROTAC. Only a relatively small number of E3 ligases out of the >600 E3 ligases encoded by human genomes have been exploited by small molecules for TPD applications.
To support PROTAC research and therapeutics, Creative BioMart provides our customers with a list of recombinant E3 proteins to effectuate TPD.
PROTAC Targets
E3 ligases
What are E3 Ligases?
E3 ligases are a class of proteins that play a crucial role in the process of ubiquitination, which is a type of protein modification. They work by recognizing specific protein substrates and facilitate the transfer of ubiquitin, a small regulatory protein, from E2 ubiquitin-conjugating enzymes to the substrate. This process controls many aspects of cell biology including protein degradation, cell cycle, DNA repair, and cell signaling. Differences or abnormalities in E3 ligases can contribute to the development of diseases, including cancer.
Applications
- Drug Discovery: PROTACs provide a new approach for drug discovery. Instead of inhibiting the action of a disease-related protein, PROTACs can completely remove it from the cell.
- Cancer Treatment: PROTACs have shown great promise in preclinical models of multiple myeloma, prostate cancer, breast cancer, and other cancers. They can degrade proteins that are typically "undruggable" by standard small molecule inhibitors.
- Neurodegenerative Diseases: PROTACs can also potentially be applied in neurodegenerative diseases by degrading proteins accumulated in the brain that contribute to conditions like Alzheimer’s, Parkinson's, and ALS.
- Antibiotic Resistance: PROTAC technology can be utilized to tackle the issue of antibiotic resistance by specifically degrading the resistance-conferring proteins in bacteria.
- Treatment of Viral Infections: PROTACs can potentially be used to degrade viral proteins, providing a new strategy for antiviral therapy.
- Research Tool: PROTACs can serve as an effective tool for biological research, helping to determine the function and importance of specific proteins within various cellular processes.
It's important to note that while PROTACs show great potential, more research is needed to determine their efficacy and safety, and overcome challenges related to tissue distribution, potential off-target effects, and identification of suitable E3 ligases.
Case Study
Case 1: Xiong Y, Zhong Y, Yim H, et al. Bridged Proteolysis Targeting Chimera (PROTAC) Enables Degradation of Undruggable Targets. J Am Chem Soc. 2022 Dec 14;144(49):22622-22632. doi: 10.1021/jacs.2c09255. Epub 2022 Nov 30. PMID: 36448571; PMCID: PMC9772293.
While many PROTACs have been developed for numerous protein targets, current small-molecule PROTAC approaches cannot target undruggable proteins that do not have small-molecule binders. Here, we present a novel PROTAC approach, termed bridged PROTAC, which utilizes a small-molecule binder of the target protein's binding partner to recruit the protein complex into close proximity with an E3 ubiquitin ligase to target undruggable proteins.
The authors discovered MS28, the first-in-class degrader of cyclin D1, which lacks a small-molecule binder. MS28 effectively degrades cyclin D1, with faster degradation kinetics and superior degradation efficiency than CDK4/6, through recruiting the CDK4/6-cyclin D1 complex to the von Hippel-Lindau E3 ligase.
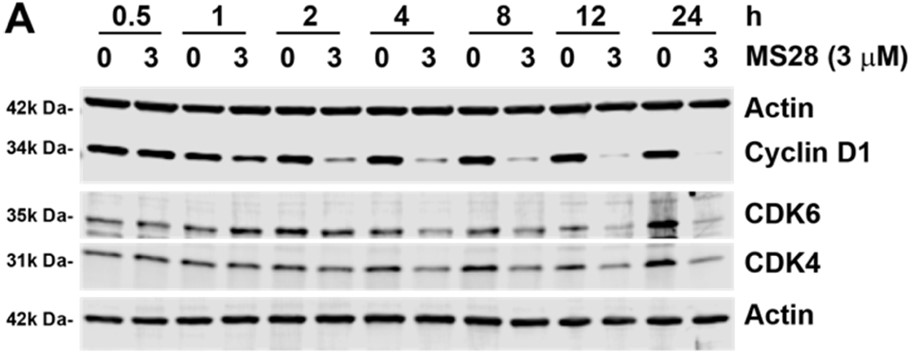
Fig1. MS28 preferentially degrades cyclin D1 over CDK4/6 in Calu-1 cells. (A) Left, MS28 degrades cyclin D1 first and CDK4/6 subsequently. Calu-1 cells were treated with DMSO or 3 μM of MS28 for the indicated time. Results shown are representative of three independent experiments. Right, quantification of relative cyclin D1, CDK4, and CDK6 abundance at each time point, following 3 μM MS28 treatment
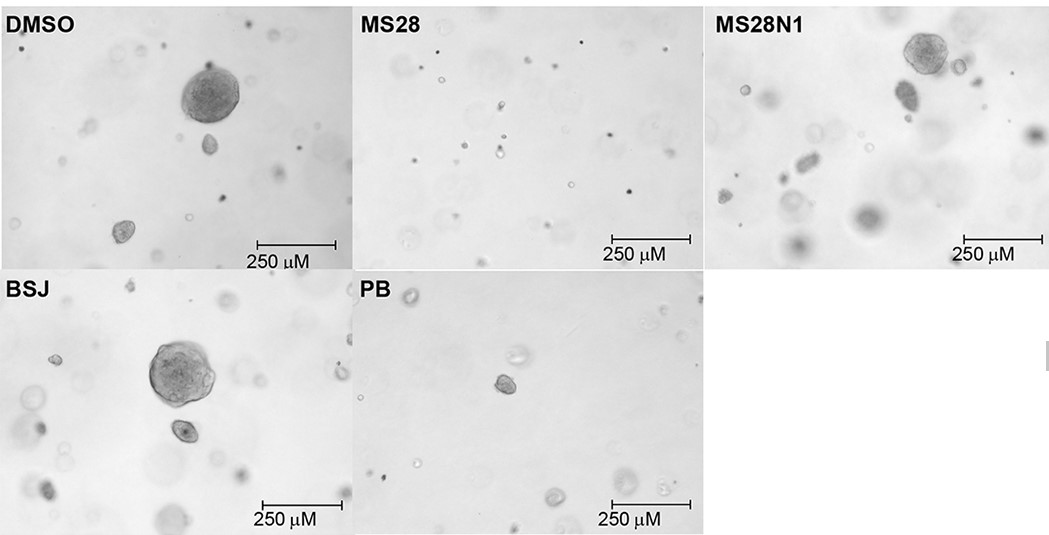
Fig2. MS28 effectively suppresses the downstream Rb-E2F pathway, proliferation, and tumorigenesis in cancer cells. MS28 suppresses clonogenicity of Calu-1 cells in a soft agar assay more effectively than PB, BSJ, and MS28N1. Images were taken at the end of the 20-day treatment. Each treatment group (at 0.3 μM) is representative of three independent experiments, each with at least five technical repeats.
Case 2: Wang W, Zhou Q, Jiang T, et al. A novel small-molecule PROTAC selectively promotes tau clearance to improve cognitive functions in Alzheimer-like models. Theranostics. 2021 Mar 11;11(11):5279-5295. doi: 10.7150/thno.55680. PMID: 33859747; PMCID: PMC8039949.
Intracellular accumulation of tau is a hallmark pathology in Alzheimer disease (AD) and the related tauopathies, thus targeting tau could be promising for drug development. Proteolysis Targeting Chimera (PROTAC) is a novel drug discovery strategy for selective protein degradation from within cells. A novel small-molecule PROTAC, named as C004019 with a molecular mass of 1,035.29 dalton, was designed to simultaneously recruite tau and E3-ligase (Vhl) and thus to selectively enhance ubiquitination and proteolysis of tau proteins.
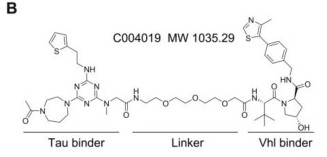
The designed C004019 composed of a tau binder, a linker and a Vhl (E3 ligase) binder with a molecular mass of 1,035.29 Dalton.
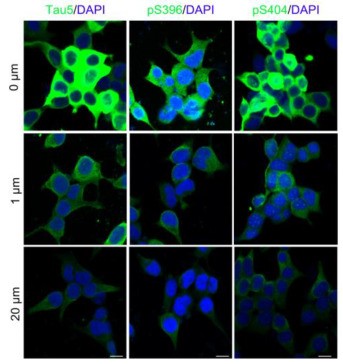
Fig1. C004019 induces tau clearance via promoting its ubiquitination and proteasome-dependent proteolysis in vitro. C004019 induced tau reduction in HEK293-hTau cells measured by immunofluorescence staining
Case 3: Cantley J, Ye X, Rousseau E, et al. Selective PROTAC-mediated degradation of SMARCA2 is efficacious in SMARCA4 mutant cancers. Nat Commun. 2022 Nov 10;13(1):6814. doi: 10.1038/s41467-022-34562-5. PMID: 36357397; PMCID: PMC9649729.
The mammalian SWItch/Sucrose Non-Fermentable (SWI/SNF) helicase SMARCA4 is frequently mutated in cancer and inactivation results in a cellular dependence on its paralog, SMARCA2, thus making SMARCA2 an attractive synthetic lethal target. The authors report the discovery of a potent and selective SMARCA2 proteolysis-targeting chimera molecule (PROTAC), A947.
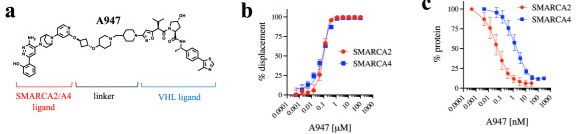
Fig1. A947 is a potent and moderately selective degrader of SMARCA2. a Chemical structure of A947. b Dose-response curves of A947 displacing a biotinylated SMARCA2/4-binding probe from recombinant SMARCA2 and SMARCA4 bromodomains. Data are presented as mean ± s.d. from 4 replicates. c Quantification of SMARCA2 and SMARCA4 protein levels by In Cell WesternTM following 20 h treatment of SW1573 cells.