Uncategorized Wednesday, 2025/05/14
Chinese Scientists Reveal Sexually Dimorphic Dopamine Circuit Determines Social Sex Preference in Mice Under Different Conditions
doi:10.1126/science.adq7001
In a new study, researchers from Xi'an Jiaotong University, Southwest Medical University, and Liaocheng University in China identified a sexually dimorphic dopaminergic circuit in the mouse brain influencing social sex preference. They found that under survival stress, the innate preference for interacting with female mice was reversed, and both male and female mice shifted towards a preference for males. The findings were published in the Science journal, under the title " Sexually dimorphic dopaminergic circuits determine sex preference."
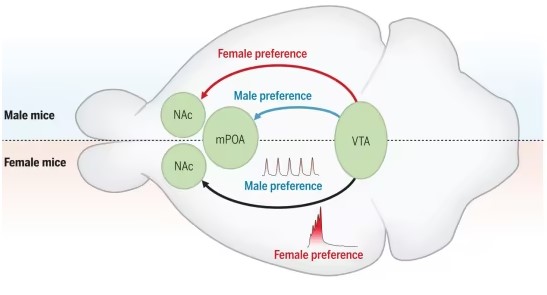
Sexually Dimorphic Dopamine Circuits Determine Social Sex Preference
In this new study, the authors examined the social sex preferences of male and female mice under normal conditions and when exposed to survival stress. Using two-color fiber photometry calcium recordings and projection-specific chemogenetic and optogenetic manipulations of dopaminergic neurons in the ventral tegmental area (VTA), they observed that a sexually dimorphic dopaminergic circuit was responsible for altering social preferences.
The results showed that under normal stress-free conditions, both male and female mice preferred interacting with female mice. However, exposure to stressors like trimethylthiazoline, contextual fear conditioning, or auditory cues led to a male preference shift.
In male mice, the proportion of time spent interacting with males increased from about 34% to around 56% with exposure. Female mice exhibited similar changes under the same conditions. Activation of VTA-DA neurons was closely associated with promoting these preference changes.
Bile Acids Impair Immunotherapy's Efficacy in Liver Cancer
doi:10.1126/science.adl4100
Immunotherapy is a modern cancer treatment approach that utilizes the patient's immune system to combat tumors. It has made a tremendous impact on treating cancers in various organ systems, including the lungs, kidneys, and bladder. However, the effects of such therapy are significantly weaker for other cancers, like liver cancer. This disparity is especially concerning as the incidence of liver cancer has nearly doubled over the past 40 years.
To understand why immunotherapy is less effective in treating liver cancer, researchers from the Salk Institute for Biological Studies closely examined how the immune system interacts with the liver in a new study. In studying mouse and human liver tumors, they found that certain bile acids in the liver affect the activity of cancer-fighting T cells. The findings were published in the Science journal, under the title " Bile acid synthesis impedes tumor-specific T cell responses during liver cancer."
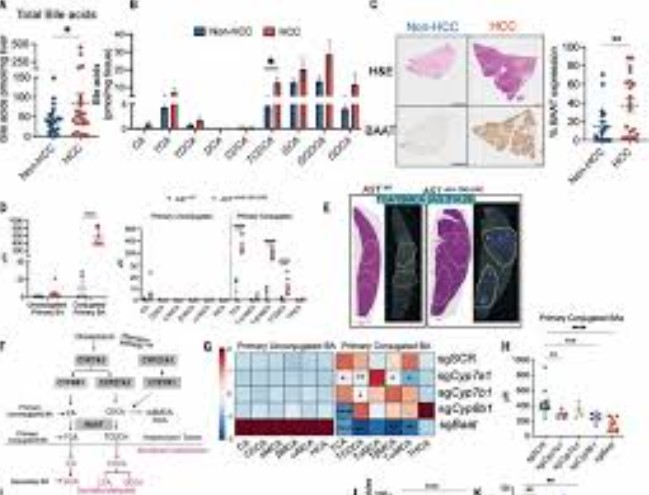
Conjugated Bile Acid Synthesis Regulates Immune Microenvironment
The authors identified several bile acids associated with impaired T cell function and tumor growth, and they successfully inhibited tumor growth and shrank existing tumors by blocking their production. They also discovered that a specific bile acid, ursodeoxycholic acid (UDCA), has a positive impact on T cell activity in the liver.
In fact, dietary supplementation to increase levels of this bile acid was sufficient to control tumor growth in mouse models of liver cancer. Since these supplements are already commercially available and used to help treat other liver diseases, the authors hope UDCA could be integrated into cancer treatment regimens, making immunotherapy more effective for these patients.
Are Immune Cells Involved in Blood Sugar Regulation?! Science Reveals Mechanism of ILC2 in Regulating Blood Sugar During Fasting and Exercise
doi:10.1126/science.adi3624
In a new study, researchers from the Champalimaud Foundation found that when the body is in a low-energy state, such as during intermittent fasting or exercise, immune cells act like couriers, passing important information between the nervous, immune, and hormonal systems to help regulate blood sugar levels. This discovery paves new pathways for treating diseases like diabetes, obesity, and cancer. The findings were published in the Science journal, under the title " Neuronal-ILC2 interactions regulate pancreatic glucagon and glucose homeostasis."
Henrique Veiga-Fernandes, the corresponding author and head of the Immunophysiology Research Laboratory at the Champalimaud Foundation, stated, "For decades, immunology has been focused on fighting infections. But we now realize that the immune system has far broader roles than we imagined." To illustrate this, he made an analogy: "If we think of our body as a big city, immune cells are not just police officers (resisting pathogens); they also play the role of couriers, passing crucial information between various 'departments'."
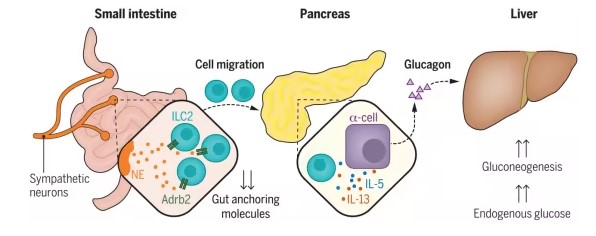
Neuroimmune interactions regulate blood glucose by controlling glucagon secretion.
Researchers conducted experiments on mice. They used genetically engineered mice lacking specific immune cells to observe their effect on blood sugar levels. They found that mice lacking a type of immune cell called ILC2 (innate lymphoid cells type 2) could not produce sufficient glucagon, resulting in low blood sugar levels. However, when ILC2 cells were transplanted into these deficient mice, their blood sugar levels normalized, demonstrating the critical role these immune cells play in stabilizing glucose levels during energy deficiency.
Our Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| IL5-4361R | Recombinant Rabbit IL5 Protein | Yeast | Rabbit | Non | 115aa |
|
| Il5-121M |
Active Recombinant Mouse Il5
|
CHO | Mouse | Non |
|
|
| IL13-4328Z | Recombinant Zebrafish IL13 Protein | Yeast | Zebrafish | Non | 141aa |
|
| IL13-370H |
Active Recombinant Human IL13
|
CHO | Human | Non | Met 1-Asn 132 |
|
| IL13-4350R | Recombinant Rabbit IL13 Protein | Yeast | Rabbit | Non | 112aa |
|
Researchers initially thought all this was regulated in the liver since glucagon acts there. However, their data pointed to a more complex process. Using advanced cell labeling techniques, they found that ILC2 cells in the gut migrate to the pancreas after fasting. This migration is not random but carefully orchestrated by the nervous system. During fasting, neurons in the gut connected to the brain release chemical signals telling ILC2 cells to leave the gut and head to the pancreas' new "postal code" within a few hours. Once there, these immune cells release cytokines—tiny chemical messengers—to instruct pancreatic cells to produce glucagon, which then signals the liver to release glucose.
Major Breakthrough! Discovery of a New Skeletal Tissue—Lipid Cartilage
doi:10.1126/science.ads9960
In a new study, an international research team led by the University of California, Irvine discovered a new type of skeletal tissue, offering tremendous potential for advancing regenerative medicine and tissue engineering. The findings were published in the Science journal, under the title "Superstable lipid vacuoles endow cartilage with its shape and biomechanics."
Most cartilage relies on the extracellular matrix for strength, but "lipocartilage" in mammalian ears, noses, and throats uniquely contains fat-filled cells called "lipochondrocytes" that provide superstable internal support, keeping the tissue soft and elastic, much like bubble wrap.
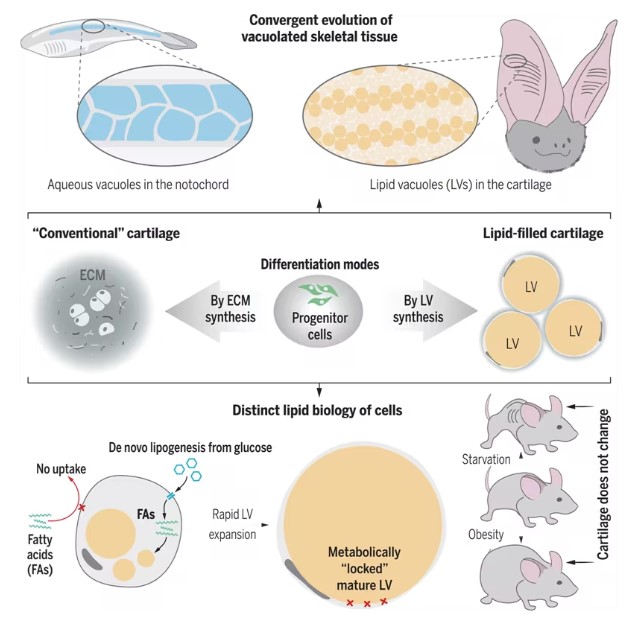
Mammalian Cartilage Filled with Lipids
This new study describes how lipochondrocytes create and maintain their lipid reservoirs and maintain a constant size. Unlike regular fat cells, lipochondrocytes do not shrink or expand with food supply.
Maksim Plikus, corresponding author and professor of developmental and cell biology at the University of California, Irvine, said, "The elasticity and stability of lipocartilage offer a compliant, resilient quality perfect for flexible body parts like earlobes or nose tips, opening exciting possibilities for regenerative medicine and tissue engineering, especially for facial defects or injuries."
New Study Challenges Classic Views on How Monkey Brains Control Natural Behaviors
doi:10.1126/science.adq6510
In a new study, researchers from the University of Parma used new telemetry equipment to record the activity of hundreds of neurons in the motor areas of monkey brains that could fully express spontaneous behaviors, such as walking, climbing, or yawning. This marks a significant advancement compared to previous studies, as existing techniques forced scientists to study stationary brains in learned and stereotyped behaviors. This new method opens up the possibility of understanding how the brain coordinates spontaneous movements in natural settings. Additionally, they revealed new mechanisms of brain control over natural behaviors, challenging some classic views on how the motor system works and opening up potential new applications for neurorehabilitation and robotics.
The findings were published in the Science journal, under the title "Neuroethology of natural actions in freely moving monkeys."
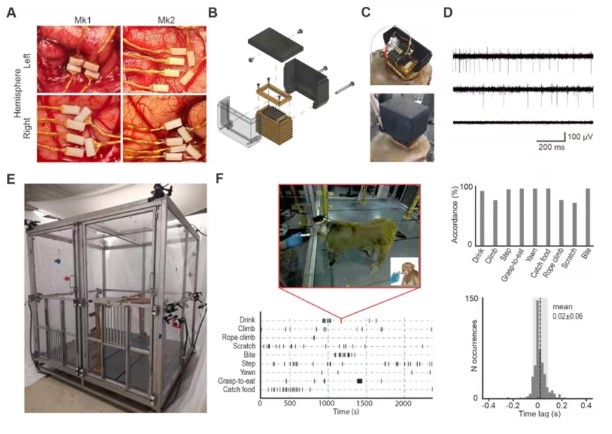
A neurobehavioral platform capable of recording from premotor neurons
Luca Bonini, corresponding author and project leader at the University of Parma, explained, "Our brain is constantly moving, and this new method changes the classic notion that specific brain areas or even single neuron cells control specific actions such as biting, drinking, or grasping."
"Based on our results, just like the keys on a piano can compose numerous different melodies, the neurons in our brain's motor area also produce complex synergies that allow us to perform the myriad of spontaneous actions we can execute, some of which have not been studied in the laboratory until now."
Rare Germline Structural Variants Increase the Risk of Pediatric Solid Tumors
doi:10.1126/science.adq0071
In a new study, researchers from various institutions, including the Dana-Farber Cancer Institute, determined that rare germline structural variants are a risk factor for non-hematologic cancers in children, including tumors in various organs. Their findings indicate that both coding and noncoding structural variants in the genome lead to childhood cancers such as neuroblastoma, Ewing sarcoma, and osteosarcoma. The findings were published in the Science journal, under the title "Rare germline structural variants increase risk for pediatric solid tumors."
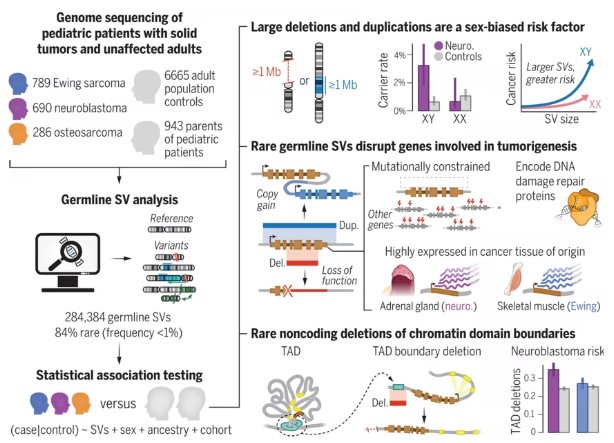
Rare Germline Structural Variants Are Risk Factors for Pediatric Solid Tumors
In this new study, the authors analyzed high-coverage germline whole-genome sequencing data of 1,765 patients with neuroblastoma, Ewing sarcoma, or osteosarcoma, 943 unaffected parents, and 6,665 unrelated adult controls, searching for hidden pathogenic genetic variants.
They developed a GATK-SV pipeline to detect large deletions, duplications, inversions, translocations, and more complex rearrangements. Applying a pan-categorical association framework systematically evaluated the structural variation in coding and noncoding regions of patients' genomes relative to controls.
The authors identified 84 broad chromosome abnormalities. These ultra-random deletions or duplications greater than a million nucleotides observed in male pediatric patients were much more frequent and presented a much higher risk of childhood cancer compared to adult controls.
Vestibulo-Ocular Reflex Maturation in Newborn Vertebrates Does Not Require Sensory Input
doi:10.1126/science.adr9982
In a new study, researchers from NYU Grossman School of Medicine focused on how vertebrates, including humans and animals from primitive fish to mammals, stabilize their gaze while moving. They discovered an ancient brain circuit translating any directional change sensed by the balance (vestibular) system in the ears into instantaneous counter-movements of the eyes, allowing them to reflexively rotate upward when a vertebrate's body tilts downward, self-regulating early in life as the animal develops.
The findings were published in the Science journal, under the title "Sensation is dispensable for the maturation of the vestibulo-ocular reflex."
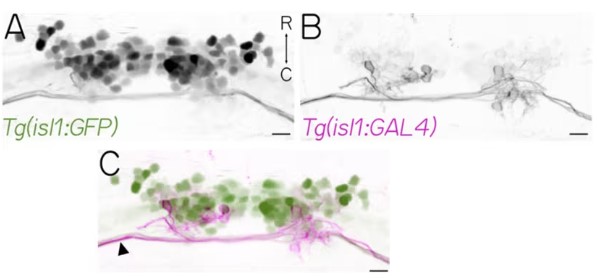
Sparse Labeling of Superior Oblique Oculomotor Neurons
This brain circuit, called the vestibulo-ocular reflex, is critical for stable perception of the surrounding environment. When disrupted by trauma, stroke, or genetic disorders, individuals may perceive the world as jumpy whenever their head or body moves. In adult vertebrates, it, along with other brain circuits, are regulated through sensory feedback from the senses (vision and balance organs). Unexpectedly, this new study found that unlike mature circuits, the vestibulo-ocular reflex circuit in newborns matures without requiring sensory input.
New Research Develops a Tool for Building Smart Cells
doi:10.1126/science.adm8485
In a new study, researchers from Rice University have developed a new construction tool for building custom sensing and response circuits in human cells. This advancement represents a major breakthrough in the field of synthetic biology, potentially revolutionizing treatments of complex diseases such as autoimmune disorders and cancer. The findings were published in the Science journal, under the title "Engineering synthetic phosphorylation signaling networks in human cells."
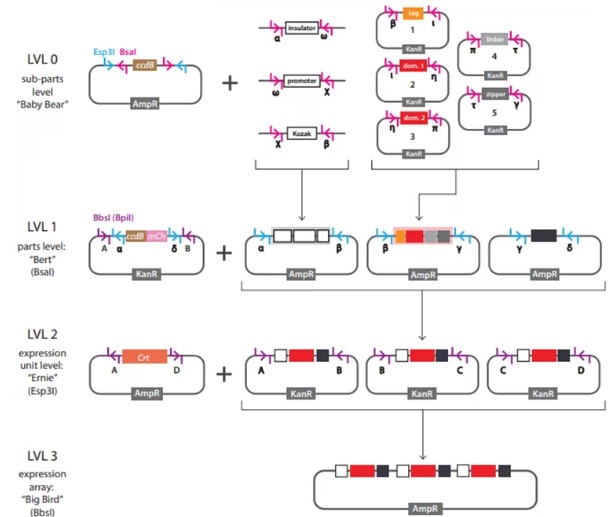
Cloning Workflow Used in This Study
Xiaoyu Yang, the first author and a graduate student in the Systems, Synthetic, and Physical Biology Ph.D. program at Rice University, said, "Imagine mini-processors composed of proteins within cells 'deciding' how to respond to specific signals like inflammation, tumor growth markers, or blood sugar levels. This study brings us closer to constructing 'smart cells' capable of detecting disease signs and immediately releasing customizable treatments."
This new method for designing artificial cell circuits relies on phosphorylation—a natural process cells use to respond to their environment, characterized by adding phosphate groups to proteins. Phosphorylation involves a range of cellular functions, including converting extracellular signals into intracellular responses, such as movement, secretions, responses to pathogens, or gene expression.
Decoding Molecular Interactions Between Therapeutic Antibodies and CD20 Using New Super-Resolution Microscopy Method
doi:10.1126/science.adq4510
In blood cancers like chronic lymphocytic leukemia, B cells of the immune system proliferate uncontrollably. A treatment approach involves marking CD20 proteins on the surface of B cells with customized antibodies. This triggers a series of immune responses that ultimately lead to the destruction of the cancer cells.
Such therapeutic antibody immunotherapies have been used to treat tumor diseases for 30 years. Professor Markus Sauer from the Biocenter of the University of Würzburg states, "Despite being crucial for the success of therapy, we still know very little about how antibodies bind to CD20 and how subsequent reactions occur."
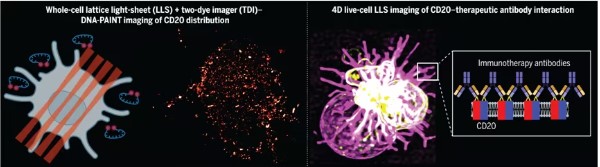
Molecular Interaction Between CD20 and Therapeutic Monoclonal Antibodies
This situation may change now. Professor Sauer and his research team developed a new super-resolution microscopy method in a new study. This enabled studying the molecular interactions between therapeutic antibodies and target molecules on tumor cells at molecular resolution in 3D for the first time. The findings were published in the Science journal on January 10, 2025, under the title "Decoding the molecular interplay of CD20 and therapeutic antibodies with fast volumetric nanoscopy," authored by Dr. Arindam Ghosh.
Our Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| CD20-4921H |
Active Recombinant Human CD20 Full Length Transmembrane protein(Nanodisc)
|
HEK293 | Human | Non | Full L. |
|
| CD20-4922H |
Active Recombinant Human CD20 Full Length Transmembrane protein(MNP)
|
HEK293 | Human | Non | Full L. |
|
| CD20-1069H | Recombinant Human CD20 protein(Glu213-Pro297), His-tagged | HEK293 | Human | His | Glu213-Pro297 |
|
| CD20-459H | Active Recombinant Human CD20 Full Length Transmembrane protein(VLPs) | HEK293 | Human | Non | Full L. Met 1 - Pro 297 |
|
This new microscopy method is called LLS-TDI-DNA-PAINT. Professor Sauer's team describes how this newly developed technology works and what discoveries have been made using it. Dr. Thomas Nerreter and Professor Martin Kortüm from Medical Clinic II at the University Hospital Würzburg also participated in this research.
Discovery of Novel Antimalarial Antibodies May Lead to Next-Generation Antimalarial Interventions
doi:10.1126/science.adr0510
In a new study, researchers from the National Institutes of Health (NIH) discovered a class of novel antibodies targeting a previously untargeted region of the malaria parasite, which may lead to new malaria prevention strategies. In animal models, they found the most effective new antibodies provide protection against the malaria parasite. They state that these antibodies are particularly promising because they bind to regions of the malaria parasite not included in current malaria vaccines, offering a potential new tool in combating this deadly disease. The findings were published in the Science journal, under the title "Protective antibodies target cryptic epitope unmasked by cleavage of malaria sporozoite protein."
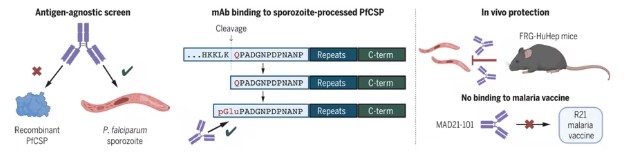
Antibodies Targeting pGlu-CSP Sites on Sporozoites Prevent Malaria
The most promising antimalarial monoclonal antibodies tested on humans so far bind to a protein called circumsporozoite protein (PfCSP) on the surface of Plasmodium falciparum sporozoites, with the binding position close to or including a repeat sequence within a region termed the central repeat of PfCSP. This part of the PfCSP protein is also included in both existing malaria vaccines. In this new study, the authors aimed to find mAbs that target new sites on the surface of P. falciparum sporozoites.
The authors employed a new approach to find new parts or epitopes on the sporozoite surface where antimalarial mAbs bind. They isolated human mAbs generated against the entire sporozoite rather than specific parts of the parasite, then tested these mAbs to see if they could neutralize sporozoites in mouse models of malaria. A human mAb named MAD21-101 was found to be most effective, protecting mice from P. falciparum infection.
This new human mAb binds to an epitope outside the conserved or similar central repeat region on PfCSP across different strains of P. falciparum. Notably, this epitope, called pGlu-CSP, is only exposed after certain developmental steps of the sporozoite, but it is broadly accessible on the sporozoite surface—suggesting, as the authors state, that if pGlu-CPS were used in a vaccine, it would effectively trigger a protective immune response.
Related Products & Services
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Immune Checkpoint Proteins
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Wei, A., Zhao, A., Zheng, C., Dong, N., Cheng, X., Duan, X., Zhong, S., Liu, X., Jian, J., Qin, Y., Yang, Y., Gu, Y., Wang, B., Gooya, N., Huo, J., Yao, J., Li, W., Huang, K., Liu, H., . . . Wang, C. (2025). Sexually dimorphic dopaminergic circuits determine sex preference. Science. https://doi.org/adq7001
- Varanasi, S. K., Chen, D., Liu, Y., Johnson, M. A., Miller, C. M., Ganguly, S., Lande, K., LaPorta, M. A., Hoffmann, F. A., Mann, T. H., Teneche, M. G., Casillas, E., Mangalhara, K. C., Mathew, V., Sun, M., Jensen, I. J., Farsakoglu, Y., Chen, T., Parisi, B., . . . Kaech, S. M. (2025). Bile acid synthesis impedes tumor-specific T cell responses during liver cancer. Science. https://doi.org/adl4100
- Šestan, M., Raposo, B., Rendas, M., Brea, D., Pirzgalska, R., Rasteiro, A., Aliseychik, M., Godinho, I., Ribeiro, H., Carvalho, T., Wueest, S., Konrad, D., & Veiga-Fernandes, H. (2025). Neuronal-ILC2 interactions regulate pancreatic glucagon and glucose homeostasis. Science. https://doi.org/adi3624
- Raul Ramos et al. Superstable lipid vacuoles endow cartilage with its shape and biomechanics. Science, 2025, doi:10.1126/science.ads9960.
- Francesca Lanzarini et al. Neuroethology of natural actions in freely moving monkeys. Science, 2025, doi:10.1126/science.adq6510.
- Riaz Gillani et al. Rare germline structural variants increase risk for pediatric solid tumors. Science, 2025, doi:10.1126/science.adq0071.
- Jayne Y. Hehir-Kwa et al. Inherited genome instability: Germline structural variants are a risk factor for pediatric extracranial solid tumors. Science, 2025, doi:10.1126/science.adu6125.
- Paige Leary et al. Sensation is dispensable for the maturation of the vestibulo-ocular reflex. Science, 2025, doi:10.1126/science.adr9982.
- Xiaoyu Yang et al. Engineering synthetic phosphorylation signaling networks in human cells. Science, 2025, doi:10.1126/science.adm8485.
- Arindam Ghosh et al. Decoding the molecular interplay of CD20 and therapeutic antibodies with fast volumetric nanoscopy. Science, 2025, doi:10.1126/science.adq4510.
- Cherrelle Dacon et al. Protective antibodies target cryptic epitope unmasked by cleavage of malaria sporozoite protein. Science, 2025, doi:10.1126/science.adr0510.
