Uncategorized Friday, 2025/06/06
This research elucidates the molecular mechanisms behind the efficacy of mRNA vaccines, emphasizing the crucial role of TENT5A in enhancing mRNA stability and immune efficacy.
Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| TENT5A-1592HFL | Recombinant Full Length Human TENT5A Protein, C-Flag-tagged | Mammalian Cells | Human | Flag | Full L. |
|
| TENT5A-2173H | Recombinant Human TENT5A Protein, His (Fc)-Avi-tagged | HEK293 | Human | Avi&Fc&His |
|
|
| Tent5a-6356M | Recombinant Mouse Tent5a Protein, Myc/DDK-tagged | HEK293 | Mouse | DDK&Myc |
|
|
| TENT5A-2173H-B | Recombinant Human TENT5A Protein Pre-coupled Magnetic Beads | HEK293 | Human |
|
In the global battle against the COVID-19 pandemic, mRNA vaccines have established a solid immune defense for humanity due to their efficient and rapid development and deployment. From Moderna to BioNTech-Pfizer, the successful application of these vaccines has not only transformed our understanding of vaccines but also inaugurated a new era for mRNA technology in the medical field. However, despite the outstanding performance of mRNA vaccines in combating the pandemic, scientists still have limited knowledge about the molecular mechanisms behind them.
Recently, a study titled “Re-adenylation by TENT5A enhances efficacy of SARS-CoV-2 mRNA vaccines” published in the international journal Nature unveils the mystery of mRNA vaccine potency. Researchers from institutions such as the International Institute of Molecular and Cell Biology in Poland reveal how the key protein TENT5A significantly bolsters the stability and immune efficacy of mRNA vaccines through the process of re-adenylation. This discovery not only offers new perspectives for optimizing mRNA vaccines but also lays the groundwork for the application of mRNA technology in treating other diseases in the future.
What is mRNA vaccine?
mRNA vaccines are a modern type of vaccine that utilize messenger ribonucleic acid (mRNA) to instruct cells to produce a specific viral protein. This protein then triggers an immune response without using live, weakened, or inactivated virus particles.
How They Work:
- mRNA Message: The vaccine contains synthetic mRNA encoding a viral antigen (e.g., the spike protein of SARS-CoV-2).
- Cell Entry: Encapsulated in lipid nanoparticles, the mRNA enters human cells and releases its genetic code.
- Protein Production: Ribosomes translate the mRNA into the viral protein, displayed on the cell surface.
- Immune Activation: The immune system recognizes the protein as foreign, producing antibodies and memory cells for future protection.
Comparison to Traditional Vaccines:
- No Live Virus: Safer for immunocompromised individuals.
- Rapid Development: Requires only the viral genetic sequence, enabling faster creation (e.g., COVID-19 vaccines developed in months).
- Production Flexibility: mRNA can be modified easily to target variants or new pathogens.
Advantages of mRNA Vaccine
- Safety: Cannot cause infection; no live virus components.
- Efficacy: High initial efficacy rates (e.g., over 90% for COVID-19 vaccines).
- Scalability: Easier to manufacture compared to traditional methods.
Challenges of mRNA Vaccine
- Cold Storage: Fragile mRNA requires ultra-low temperatures (e.g., -70°C for Pfizer-BioNTech).
- Novelty: First widespread use during COVID-19; long-term data on efficacy and safety are emerging.
- Myths Addressed: mRNA does not alter human DNA, as it remains in the cytoplasm and degrades quickly.
mRNA vaccines activate the human immune system by encoding viral proteins, but the metabolic processes they undergo within cells, particularly the dynamic changes in the poly(A) tail, have long been a challenge for researchers. The length of the poly(A) tail directly influences mRNA stability and translation efficiency, subsequently affecting vaccine efficacy. This study aims to elucidate the metabolic mechanisms of mRNA vaccines within cells, especially the changes in the poly(A) tail and how these changes impact the vaccine's immune efficacy.
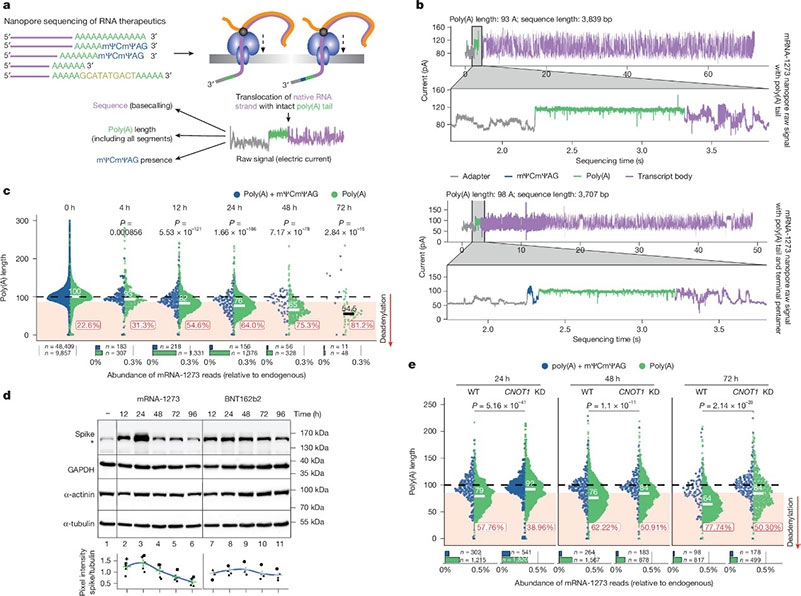
Poly(A) Tail Elongation in Macrophages in vitro and in vivo
Researchers focused on Moderna's mRNA-1273 and BioNTech-Pfizer's BNT162b2 vaccines, employing nanopore sequencing technology to analyze individual mRNA molecules with an emphasis on the dynamic changes of their poly(A) tails. The experiments involved various cell lines, including HEK293T, A549, mouse bone marrow-derived macrophages (mBMDMs), and human monocyte-derived macrophages (hMDMs). Through transfection of vaccine mRNA into these cells and sequencing analysis of samples collected at different time points, the researchers detailed the metabolic process of mRNA vaccines within cells.
The study found that the poly(A) tail of mRNA-1273 undergoes rapid degradation within cells, but is simultaneously re-adenylated by the TENT5A protein, thereby extending its length and enhancing stability. This re-adenylation is particularly pronounced in macrophages and is closely linked to vaccine immune efficacy. In contrast, BNT162b2 showed lower re-adenylation efficiency, potentially due to its lower membrane association.
The Crucial Role of TENT5A in Vaccine Efficacy
Researchers also discovered that the expression of TENT5A is significantly upregulated in macrophages at the vaccine injection site, indicating its critical role in vaccine-induced immune responses. To further verify the role of TENT5A, they conducted experiments knocking out the TENT5A and TENT5C genes, resulting in significant reductions in the stability and poly(A) tail length of mRNA-1273. Additionally, using mouse models, they observed that TENT5A knockout mice generated significantly lower levels of anti-spike protein IgG post-vaccination compared to wild-type mice, further confirming the key role of TENT5A in enhancing vaccine immune efficacy.
Significance and Future Prospects of the Study
This study is the first to reveal the metabolic mechanism of mRNA vaccines within cells, particularly the TENT5A-mediated re-adenylation process. This discovery not only enriches our understanding of mRNA metabolism but also provides new strategies for optimizing mRNA vaccine design. By enhancing TENT5A activity or designing mRNA structures more easily recognized by TENT5A, future researchers may improve mRNA vaccine stability and immune efficacy further.
Moreover, this mechanism opens new avenues for applying mRNA technology to other disease treatments, such as enhancing antigen expression to activate the host immune system in cancer therapies. With ongoing exploration of mRNA technology by scientists, it is reasonable to believe that mRNA vaccines will play a more significant role in future medical fields.
Related Products & Services
- SARS-CoV-2 Proteins and Their Target Proteins
- PROTAC Targets
- Cell and Gene Therapy
- Immune Checkpoint Proteins
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Krawczyk, P.S., Mazur, M., Orzeł, W. et al. Re-adenylation by TENT5A enhances efficacy of SARS-CoV-2 mRNA vaccines. Nature (2025). doi:10.1038/s41586-025-08842-1
