Uncategorized Sunday, 2025/07/13
The chemokine receptor CXCR4 partial agonist TFF2-MSA can reduce the generation of immunosuppressive neutrophils at the source, thereby synergizing with PD-1 inhibitors to suppress primary tumor growth and distant metastasis, and prolong the survival of gastric cancer mouse models.
We witnessed the power of tumor's "long - arm jurisdiction" last month.
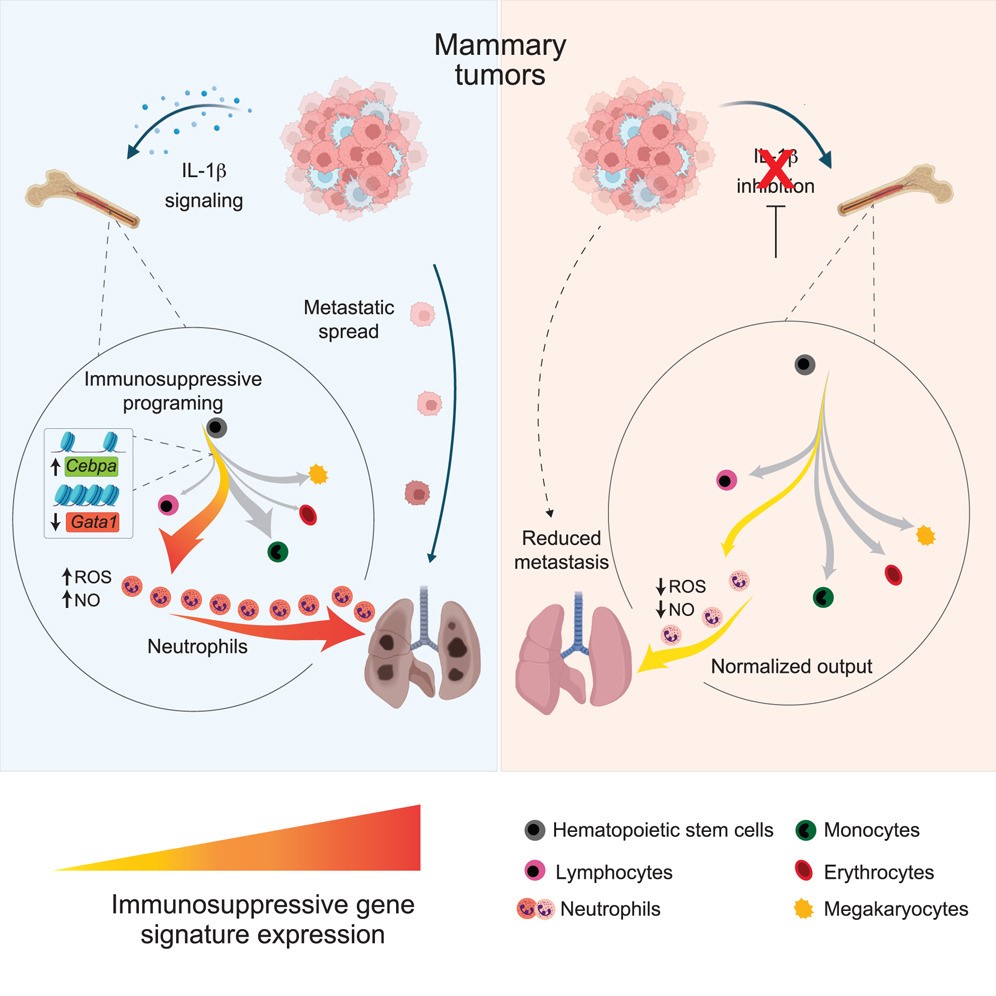
Early last month, researchers from the Netherlands Cancer Institute found that breast cancer's remodeling of neutrophils starts with hematopoiesis in the bone marrow, leading to the generation of numerous immunosuppressive neutrophils, suppressing anti - tumor immunity and promoting breast cancer metastasis.
In fact, breast cancer isn't the only one. Stomach and other cancers can do this too.
Evidently, inhibiting the generation of immunosuppressive neutrophils or breaking the tumor's "long - arm jurisdiction" could enhance anti - tumor immunity and improve immunotherapy outcomes.
Many scientists are seeking breakthroughs. A month ago, researchers from the Netherlands Cancer Institute found anti - IL - 1β antibodies promising.
Today, the research team led by Timothy C. Wang from Columbia University Irving Medical Center in the US also published an important paper in the prestigious journal Cancer Cell, announcing a new direction.
Related Proteins
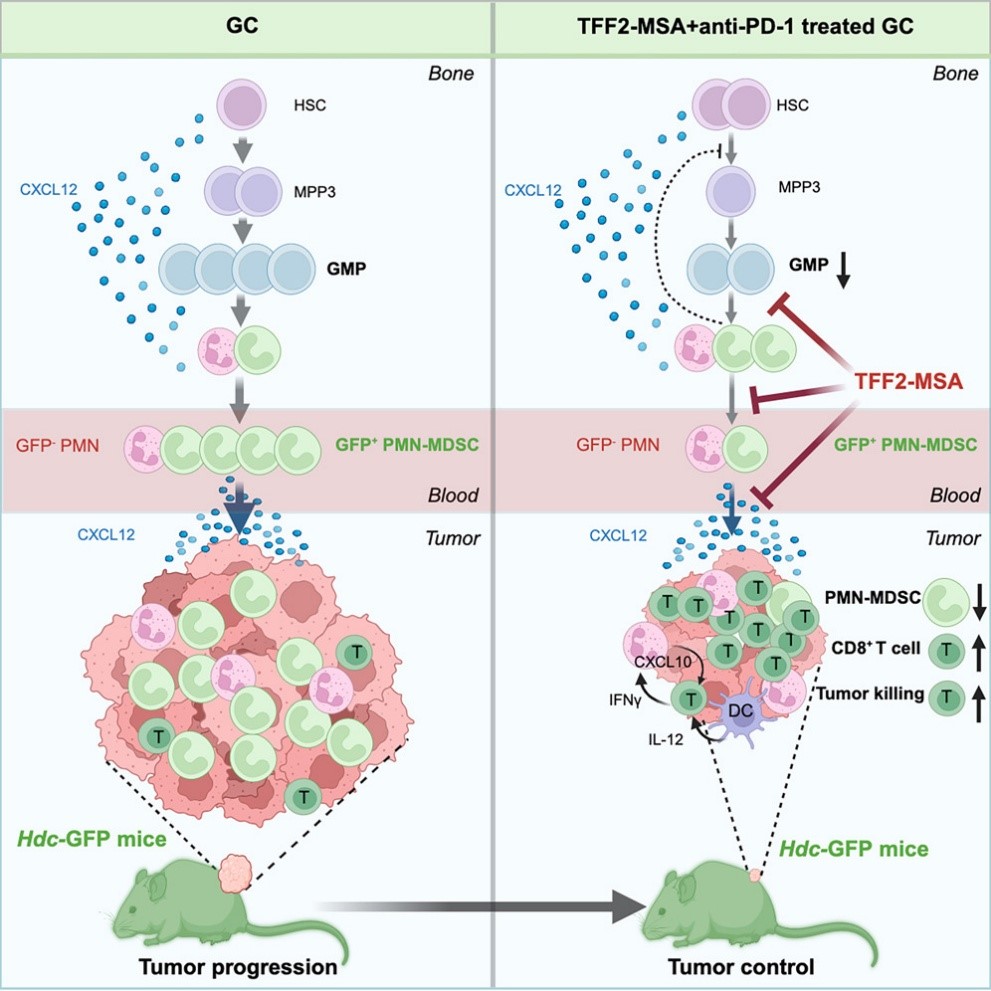
They discovered that the chemokine receptor CXCR4 partial agonist TFF2-MSA can reduce the generation of immunosuppressive neutrophils at the source and synergize with PD-1 inhibitors to suppress primary tumors and distant metastasis, prolonging survival in gastric cancer mouse models.
The Wang team chose CXCR4 as the breakthrough because many solid tumors upregulate chemokine CXCL12, using the CXCL12 - CXCR4 axis to recruit polymorphonuclear myeloid - derived suppressor cells (PMN-MDSCs, pathologically activated neutrophils) and exclude T cells, creating an immunosuppressive microenvironment.
Thus, CXCR4 antagonists blocking the CXCL12 - CXCR4 axis have been used in clinical trials with immune checkpoint inhibitors. Unfortunately, these efforts had limited therapeutic success, possibly due to CXCR4's role in maintaining bone marrow homeostasis and immune function. Some studies also found that overexpressing or supplementing CXCL12 can inhibit tumor progression, indicating that some CXCR4 signaling may benefit anti - tumor immunity.
It's clear that the CXCL12 - CXCR4 axis has broad effects and multifaceted functions, being exploited by tumors to promote cancer but also having anti - cancer potential. Based on this, the Wang team proposed the partial agonist anti - cancer approach. As the name suggests, partial agonists bind to CXCR4 but activate signals weaker than full agonists, essentially weakening CXCL12 - CXCR4 axis signaling.
Scientists had already identified TFF2 as a CXCR4 partial agonist, but similar studies were lacking. So, TFF2 became a readily available option. To target PMN-MDSCs, the Wang team fused TFF2 with mouse serum albumin (MSA), developing the stabilized peptide TFF2-MSA.
With TFF2-MSA ready, the Wang team tested its monotherapy and combination with immune checkpoint inhibitors in various cancer mouse models.
In the ACKP gastric cancer model, neither PD-1 inhibitors nor TFF2-MSA alone worked, but the combination showed strong synergy, effectively suppressing tumor growth. In gastric cancer liver metastasis and spontaneous lung metastasis models, TFF2-MSA monotherapy reduced metastases by 59%, while combining with PD-1 inhibitors reduced them by over 90% and nearly doubled median survival.
Moreover, TFF2-MSA enhanced PD-1 inhibitors' efficacy in colon and pancreatic cancer models and improved Lag - 3 inhibitors' effectiveness in ACKP gastric cancer models, indicating broad applicability.
Does TFF2-MSA work by reducing immunosuppressive PMN-MDSCs in the tumor microenvironment?
By fluorescently labeling PMN-MDSCs, the Wang team confirmed that TFF2-MSA selectively reduces immunosuppressive PMN-MDSCs there. Notably, with PMN-MDSCs' decrease, there was an increase in polymorphonuclear leukocytes (mainly neutrophils) enriched in antigen - presentation and interferon - stimulated genes, as well as in T cells and NK cells.
In other words, after TFF2-MSA treatment, the tumor microenvironment shifts from immunosuppressive to immunostimulatory.
In follow - up studies, the Wang team confirmed that TFF2-MSA acts as a CXCR4 partial agonist. Its induced CXCR4 receptor internalization was weaker than that of agonist CXCL12. Also, TFF2-MSA weakly activated AKT signaling via CXCR4 and reduced CXCL12 - mediated AKT overactivation. When knocking out the CXCR4 - encoding gene in mouse myeloid cells, the tumor - inhibiting effect of TFF2-MSA combined with immune checkpoint inhibitors disappeared.
Remarkably, TFF2-MSA not only reduces PMN-MDSCs in the tumor microenvironment but also intervenes at the hematopoietic stage, systemically suppressing cancer - induced PMN-MDSC generation.
Regarding whether the mechanisms of this finding exist in cancer patients, the Wang team studied gastric cancer patient samples. They found that compared to healthy donors, untreated gastric cancer patients had higher PMN-MDSC levels in peripheral blood and lower plasma TFF2 levels. Plasma TFF2 levels negatively correlated with total PMN-MDSCs, and low TFF2 expression in gastric cancer tissue linked to poor overall survival. This suggests similar mechanisms may exist in gastric cancer patients.
In summary, Timothy C. Wang's team's research identifies a novel drug regulating PMN-MDSC levels. Combined with immune checkpoint inhibitors, it may systemically improve the immunosuppressive environment in gastric cancer patients and enhance the therapeutic efficacy of immune checkpoint inhibitors.
Related Products & Services
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Immune Checkpoint Proteins
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Garner H, Martinovic M, Liu NQ, et al. Understanding and reversing mammary tumor-driven reprogramming of myelopoiesis to reduce metastatic spread. Cancer Cell. Published online May 5, 2025. doi:10.1016/j.ccell.2025.04.007
- Qian et al., A CXCR4 partial agonist improves immunotherapy by targeting immunosuppressive neutrophils and cancer-driven granulopoiesis, Cancer Cell (2025), https://doi.org/10.1016/j.ccell.2025.06.006
- Dubeykovskaya Z, Dubeykovskiy A, Solal-Cohen J, Wang TC. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J Biol Chem. 2009;284(6):3650-3662. doi:10.1074/jbc.M804935200

