Uncategorized Sunday, 2025/07/13
Nature: HIV Viral Protein Nef Empowers Novel CAR-T Cells to Create Off-the-Shelf Donor Cells Ready for Immediate Use
DOI: 10.1038/s41586-025-08657-0
CAR-T cell therapy is one of the most promising new cancer therapies to emerge in recent years. It involves extracting a patient's own T cells and genetically modifying them to recognize specific targets on the surface of cancer cells. A major limitation of this autologous CAR-T cell therapy, which uses the patient's own T cells, is that the cells must be customized for each treatment. This means patients must wait for their T cells to be modified before infusion—a luxury of time they may not have.
Now, in a new study, researchers from the Memorial Sloan Kettering Cancer Center (MSK) have made new progress in this field, potentially allowing the use of ready-made CAR-T cells derived from healthy donors and stored for immediate availability when patients need them. They identified a method to modify donor CAR-T cells, also known as allogeneic CAR-T cells, so that they are not rejected by the recipient patients and can continue to fight cancer.
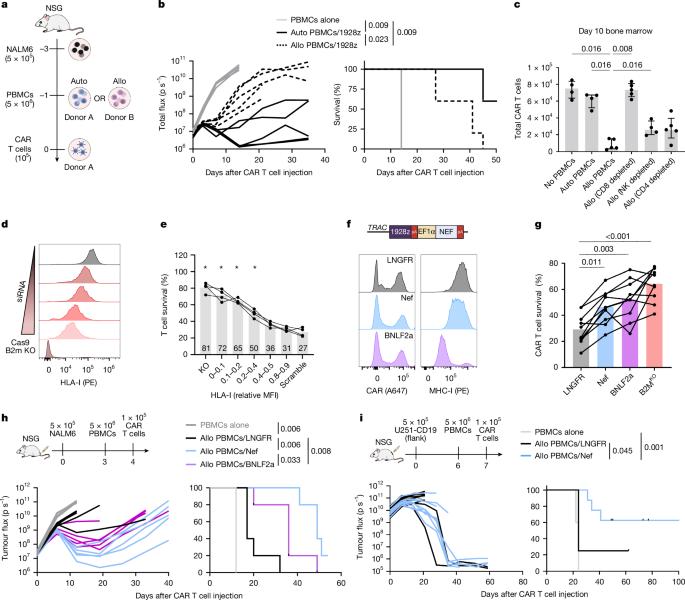
Fig5. Viral evasin HLA-I reduction protects against allogeneic CD8+ T cell killing
The study results were published online in Nature, in a paper titled " HIV immune evasin Nef enhances allogeneic CAR T cell potency." The research was conducted in the laboratory of Dr. Michel Sadelain, a pioneer in CAR-T cell therapy.
This new approach involves equipping CAR-T cells with a protein called Nef. The authors found that inserting Nef into donor CAR-T cells in cancer mouse models enabled these cells to survive and maintain potency. Karlo Perica, the first author of the paper and a medical doctor at Memorial Sloan Kettering Cancer Center, said, "This may represent an important step toward creating safe and effective allogeneic CAR-T cells, which could significantly increase the number of patients who can benefit from this immunotherapy."
New Breakthrough in CAR-T Cell Therapy! Nat Commun: CUL5 Gene Modification May Promote T Cell Growth and Improve the Success Rate of Cancer Therapy
DOI: 10.1038/s41467-024-54794-x
Chimeric antigen receptor (CAR) T cell therapy is a promising cancer treatment, but enhancing its efficacy has been an enigma. Recently, a research report published in Nature Communications titled "Cullin-5 deficiency promotes chimeric antigen receptor T cell effector functions potentially via the modulation of JAK/STAT signaling pathway" revealed a method to improve the effectiveness of this potential cancer therapy. Researchers from Nagoya University in Japan and other institutions discovered that modifying a specific gene could enhance the ability of immune cells to combat cancer over an extended period, potentially reducing the likelihood of cancer recurrence.
Researchers noted that reducing the activity of the CUL5 gene could enhance the anticancer effects of CAR-T cells, thereby improving treatment outcomes for patients with various aggressive cancers such as leukemia, lymphoma, and multiple myeloma. CAR-T cell therapy is an innovative treatment for advanced cancers where scientists genetically modify a patient's immune T cells in the laboratory to incorporate chimeric antigen receptors (CARs), enabling these cells to target and eliminate cancer cells. In this study, the researchers focused on CARs targeting the CD19 protein on the surface of B cells, as B cells are the specific immune cell type that undergoes malignant transformation in diseases like leukemia.
Related Proteins
Despite clinicians having successfully treated many patients with this therapy, many still cancer experience recurrence due to the hostile environment created by cancerous cells. After repeated exposure to cancer cells, CAR-T cells lose their ability to proliferate and effectively attack tumors.
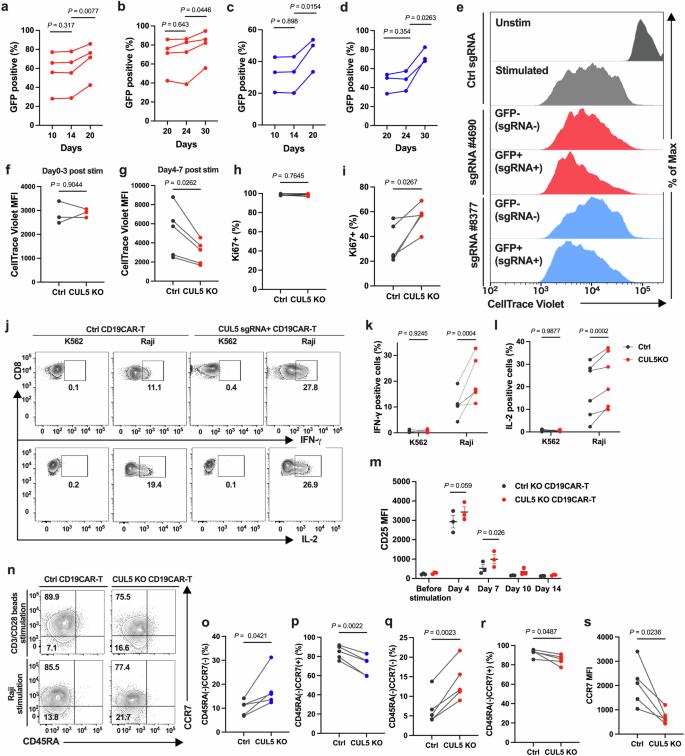
Fig6. CUL5 knockout (KO) promotes sustained proliferation and enhances effector functions later after stimulation.
To address this issue, researchers utilized CRISPR screening technology to identify genes that could enhance the efficacy of CAR-T cell therapy. CRISPR allows scientists to switch off individual genes in cells and observe which genes are crucial for specific functions. The results indicated that the CUL5 gene is involved in the degradation of certain proteins within cells. When CUL5 is inactive, the JAK-STAT signaling pathway becomes more sustained. This pathway sends signals encouraging T cell growth and proliferation, enabling a better response to cancer.
Mol Ther Subjournal: New Breakthrough in CAR Treg Cells Brings Hope for Autoimmune Diseases!
DOI: 10.1016/j.omtm.2024.101385
In a new study, researchers from the Hollings Cancer Center at the Medical University of South Carolina unveiled new rules for designing chimeric antigen receptor (CAR) Treg cells. This breakthrough may provide more effective tools for combating autoimmune diseases such as type 1 diabetes. The findings were recently published in Molecular Therapy - Methods & Clinical Development in a paper titled "High-affinity chimeric antigen receptor signaling induces an inflammatory program in human regulatory T cells."
CAR-T cell therapy has achieved significant success in blood cancer treatment. This therapy involves adding CARs to a patient's own T cells, enabling them to recognize and attack specific cancer cells. However, when it comes to autoimmune diseases, the situation becomes more complex. To effectively treat these diseases, Treg cells must be engineered to be more precise and efficient.
In this study, the researchers identified a key issue: the currently used CAR constructs are not tailored to the biological characteristics of Treg cells, causing these cells to produce unnecessary inflammatory signals, which contradicts their original goal of alleviating inflammation.
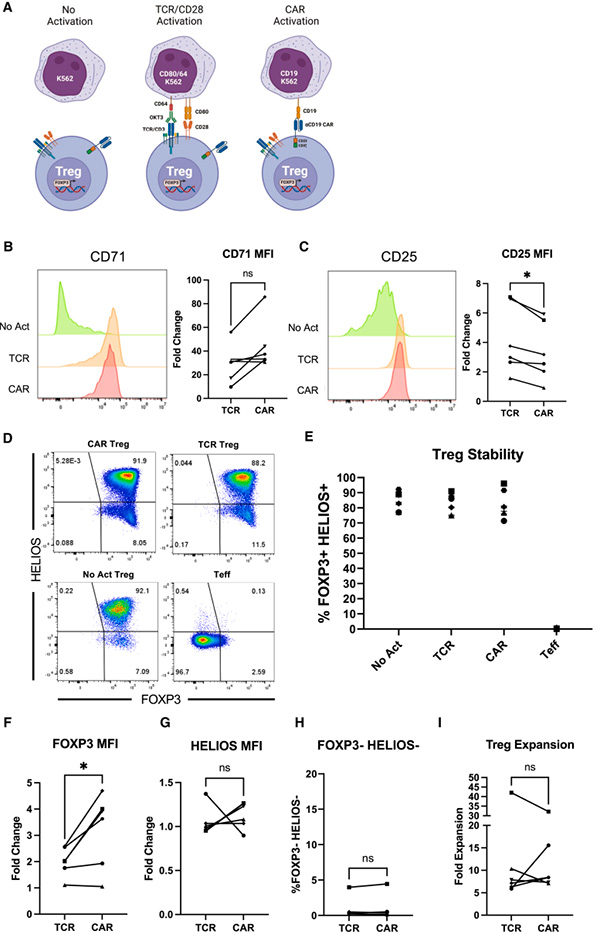
Fig7. CAR and endogenous TCR/CD28 activation result in phenotypically similar Tregs
First author Russell Cochrane said, "We attempted to address this issue by reducing the affinity of CARs—that is, their binding strength to targets. We found that it can still suppress immune responses through various mechanisms while reducing pro-inflammatory consequences."
Dr. Leonardo Ferreira, the corresponding author of the paper, stated, "This study provides new clues for realizing the promise of Treg cell therapy. We know it is possible, and we will continue to work on it." Researchers are exploring different target selection strategies to better adapt to various types of diseases. For example, in organ transplantation, the goal is to enable the immune system to function normally rather than broadly suppressing the entire immune system as traditionally done.
Haematologica: New Study Assesses the Risk of Complications from CAR-T Cell Therapy Tisa-cel
DOI: 10.3324/haematol.2024.286169
Chimeric antigen receptor (CAR) T cell therapy is a form of cancer immunotherapy. In this immunotherapy, scientists collect T cells from patients and genetically modify them to produce CARs that recognize specific targets on the surface of cancer cells, enabling these T cells to locate and destroy cancer cells.
CAR-T cell therapy has shown promising results in treating relapsed or refractory B-cell lymphoma. In a new study, researchers from Juntendo University in Japan, including Professor Jun Ando, Professor Miki Ando, and Dr. Erina Hosoya, explored the risks associated with CAR-T cell therapy. The findings were recently published in Haematologica in a paper titled "Eleven cases of laryngeal edema after tisagenlecleucel infusion: a 3-year single center retrospective study of CD19-directed chimeric antigen receptor T-cell therapy for relapsed and refractory B-cell lymphomas."
Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| CD19-3308HF |
Active Recombinant Human CD19 Protein, Fc-tagged, FITC conjugated
|
HEK293 | Human | Fc | 20-291 a.a. | |
| Cd19-3308MAF488 |
Active Recombinant Mouse Cd19 Protein, His-tagged, Alexa Fluor 488 conjugated
|
HEK293 | Mouse | His | Met1-Gly287, 282 | |
| Cd19-3308MAF555 |
Active Recombinant Mouse Cd19 Protein, His-tagged, Alexa Fluor 555 conjugated
|
HEK293 | Mouse | His | Met1-Gly287, 282 | |
| Cd19-3308MAF647 |
Active Recombinant Mouse Cd19 Protein, His-tagged, Alexa Fluor 647 conjugated
|
HEK293 | Mouse | His | Met1-Gly287, 282 | |
| Cd19-3308MF |
Active Recombinant Mouse Cd19 Protein, His-tagged, FITC conjugated
|
HEK293 | Mouse | His | Met1-Gly287, 282 | |
| CD19-3309HA |
Active Recombinant Human CD19 protein, His-tagged, APC labeled
|
HEK293 | Human | His | Met1-Lys291 | |
| CD19-559H |
Active Recombinant Human CD19 Protein, His-Avi-tagged, Biotinylated
|
HEK293 | Human | Avi&His | Pro 20 - Lys 291 |
Dr. Hosoya said, "We conducted a single-center retrospective analysis of 59 patients with relapsed/refractory B-cell lymphoma who received tisagenlecleucel (tisa-cel) CAR-T cell therapy between September 2020 and September 2023. Among the 41 study patients (38 with diffuse large B-cell lymphoma (DLBCL) and 3 with follicular lymphoma (FL)), 37 DLBCL patients and 1 FL patient completed response assessments."
The authors tracked overall survival (OS) and progression-free survival (PFS) over 12 months and found OS to be 73.8% and PFS to be 49.6%. Safety monitoring revealed that 30 of the 41 patients treated with tisa-cel developed cytokine release syndrome (CRS), an inflammatory side effect.
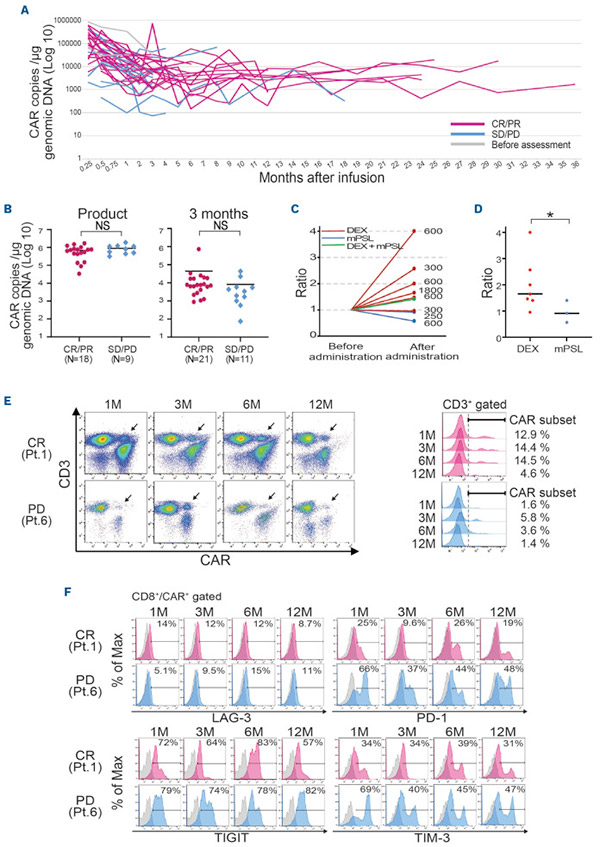
Fig8. Long-term monitoring of the chimeric antigen receptor (CAR) transgene and impact of steroid treatment on the transgene.
Among the 30 patients with CRS, 14 experienced more severe CRS (grade 3 or higher) and tended to receive higher doses of CAR-positive cells, leading to earlier fever onset compared to patients with milder CRS (grade 1 or 2).
The authors identified 11 cases of laryngeal edema, a serious and previously unrecognized side effect of CAR-T cell therapy. Initially thought to affect only patients with neck tumors, this condition was found in all patients regardless of tumor location. Due to airway obstruction caused by laryngeal edema, intensive care and airway management were provided. This condition typically appeared within 3.4 days after infusion and resolved in all patients within 14 days.
Dr. Hosoya stated, "Although there have been some case reports of laryngeal edema in patients treated with tisa-cel for B-cell lymphoma, the mechanism underlying this phenomenon remains unknown. Moreover, despite the significant risk of life-threatening airway obstruction, there are currently no established management guidelines. Therefore, we assessed the risk factors for laryngeal edema and the impact of steroid therapy on CAR-T cell expansion and clinical outcomes."
Mol Ther: A Novel Viral Vector Platform Enables More Efficient Manufacturing of CAR-T Cells
DOI: 10.1016/j.ymthe.2024.12.012
In a new study, Associate Professor Leszek Liowski from the Child Health Research Institute at the Sydney Medical and Health School and his team identified a new method for producing therapeutic products—chimeric antigen receptor (CAR) T cells—that could improve cancer treatment. The findings were recently published in Molecular Therapy in a paper titled "Tailoring Capsid-Directed Evolution Technology for Improved AAV-Mediated CAR-T Generation."
In recent years, advances in CAR-T cell technology have included the use of novel viral vectors to deliver functional CARs to patients' T cells to produce therapeutic CAR-T cell products. Adeno-associated virus (AAV) vectors, known for their inherent safety, represent the next frontier for directly delivering therapeutic genes to patients in CAR-T cell products.
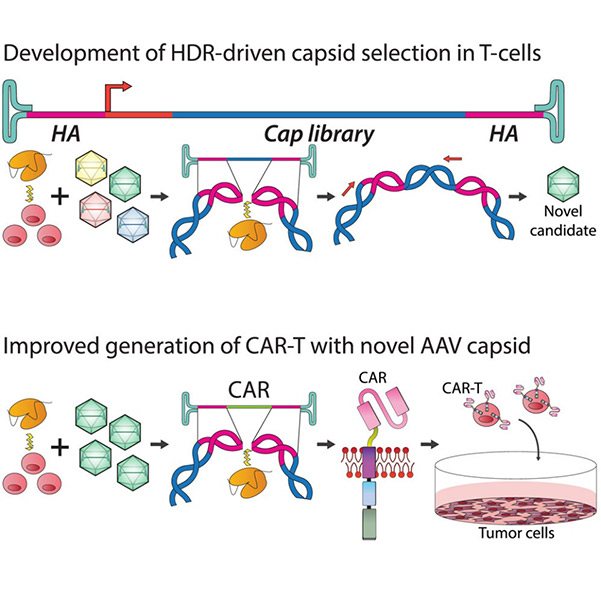
Fig9. Tailoring capsid-directed evolution technology for improved AAV-mediated CAR-T generation graphical abstract
In this study, Associate Professor Liowski and his team, including co-author Adrian Westhaus, developed a new approach to construct customized AAV variants capable of more effectively targeting patients' T cells, positioning them as next-generation AAV vectors for CAR-T cell product development.
In this proof-of-concept study, Liowski's team demonstrated that significantly reduced viral vector doses could not only lower the cost of future CAR-T cell products but also confirm that therapeutic CAR-T cells developed using these new AAVs kill cancer cells with higher efficiency. This finding suggests that the new therapy could also enhance the therapeutic efficacy of the treatment.
Nat Immunol: A Combination of Three Drugs May Enhance the Efficacy of CAR-T Cell Therapy
DOI: 10.1038/s41590-024-02042-1
Chimeric antigen receptor T cells (CAR-T cells) possess phenotypic characteristics similar to T stem cells (TSCM), promoting sustained antitumor effects. Recently, a research report published in Nature Immunology titled "A Multi-Kinase Inhibitor Screen Identifies Inhibitors Preserving Stem-Cell-Like Chimeric Antigen Receptor T Cells" identified a combination of three different drugs through a preclinical study. This combination could be used to generate more potent immune system CAR-Ts (chimeric antigen receptor T cells) to combat cancer, and this finding has significant implications for improving the production of clinically used CAR-T cells.
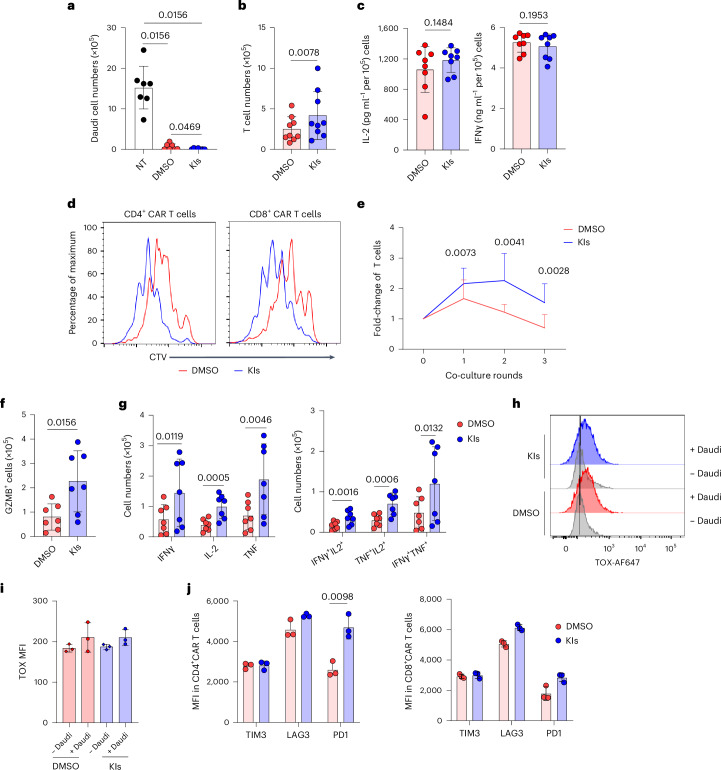
Fig10. KIs enhance antitumor activity of CAR.CD19 T cells in vitro.
CAR-T cell immunotherapy involves collecting T cells from a patient's immune system, genetically engineering them in the laboratory, and reinfusing them into the patient to recognize targets on the surface of cancer cells. Patients treated with certain types of CAR-T cell therapy often show significant responses to cancer, especially when the reengineered cells contain immune cell subsets recognizable by T memory stem cells (TSCM).
Professor Gianpietro Dotti noted that producing CAR-T cells for reinfusion in the laboratory may vary greatly among different patients. The absence of certain cell types in the final product can significantly reduce the cells' ability to persist long term. The study results suggest that identifying additional drugs during the reengineering process could help preserve key cell subsets responsible for long-term persistence.
Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| ITK-3745H | Recombinant Human ITK protein, His-tagged | E.coli | Human | His | 151-258 aa | |
| ITK-1419H |
Active Recombinant Human ITK, GST-tagged
|
Sf9 Cells | Human | GST | 352 aa-end |
|
| ADCK3-1339M | Recombinant Mouse ADCK3 Protein | Mammalian Cells | Mouse | His |
|
|
| ADCK3-239R | Recombinant Rhesus monkey ADCK3 Protein, His-tagged | Mammalian Cells | Rhesus macaque | His |
|
|
| MAP3K4-5332M | Recombinant Mouse MAP3K4 Protein, His (Fc)-Avi-tagged | HEK293 | Mouse | Avi&Fc&His |
|
|
| CDK13-01H | Recombinant Human CDK13/Cyclin K protein, GST-tagged | Insect Cells | Human | GST | CDK13 (516-end) and Cyclin K (Full Length) |
|
| CDK13-3193M | Recombinant Mouse CDK13 Protein | Mammalian Cells | Mouse | His |
|
In this study, researchers used laboratory experiments and mouse studies to identify several kinases involved in the enrichment of TSCM-like CAR-T cells, including ITK, ADCK3, MAP3K4, and CDK13. In particular, ADCK3 and MAP3K4 emerged as potential novel targets within T cells. This indicates that further in-depth research is needed to uncover how T cells differentiate to perform important immune functions.
Related Products & Services
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Immune Checkpoint Proteins
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Engineering TCR-controlled fuzzy logic into CAR T cells enhances therapeutic specificity. Kondo, Taisuke et al. Cell, Volume 188, Issue 9, 2372 - 2389.e35
- Engineering sonogenetic EchoBack-CAR T cells. Liu, Longwei et al. Cell, Volume 188, Issue 10, 2621 - 2636.e20
- Aïda Falgàs, Rodrigo Lázaro-Gorines, Samanta Romina Zanetti, et al.; A TIM-3–Fc decoy secreted by engineered T cells improves CD19 CAR T-cell therapy in B-cell acute lymphoblastic leukemia. Blood 2025; 145 (22): 2599–2613. doi: https://doi.org/10.1182/blood.2024025440
- Braun, T., Rade, M., Merz, M., Klepzig, H., Große, F., Fandrei, D., Pham, N., Kreuz, M., Kuhn, C. K., Kuschel, F., Löffler, D., et al. (2025). Multiomic profiling of T cell lymphoma after therapy with anti-BCMA CAR T cells and GPRC5D-directed bispecific antibody. Nature Medicine, 31(4), 1145-1153. https://doi.org/10.1038/s41591-025-03499-9
- Perica K, Kotchetkov IS, Mansilla-Soto J, et al. HIV immune evasin Nef enhances allogeneic CAR T cell potency. Nature. 2025 Apr;640(8059):793-801. doi: 10.1038/s41586-025-08657-0. Epub 2025 Jan 30. PMID: 39884316.
- Adachi, Y., Terakura, S., Osaki, M., et al. (2024). Cullin-5 deficiency promotes chimeric antigen receptor T cell effector functions potentially via the modulation of JAK/STAT signaling pathway. Nature Communications, 15(1), 1-14. https://doi.org/10.1038/s41467-024-54794-x
- Cochrane RW, Robino RA, Granger B, et al. High-affinity chimeric antigen receptor signaling induces an inflammatory program in human regulatory T cells. Mol Ther Methods Clin Dev. 2024 Nov 18;32(4):101385. doi: 10.1016/j.omtm.2024.101385. PMID: 39687729; PMCID: PMC11647616.
- Hosoya E, Ando J, Kinoshita S, Furukawa Y, Toyoshima Y, Azusawa Y, Mitsumori T, Sato E, Takano H, Tsukune Y, Watanabe N, Takaku T, Yasuda H, Hamano Y, Sasaki M, Nojiri S, Ishii M, Ando M. Eleven cases of laryngeal edema after tisagenlecleucel infusion: a 3-year single center retrospective study of CD19-directed chimeric antigen receptor T-cell therapy for relapsed and refractory B-cell lymphomas. Haematologica. 2025 Mar 1;110(3):777-783. doi: 10.3324/haematol.2024.286169. PMID: 39415689; PMCID: PMC11873693.
- Westhaus A, Barba-Sarasua E, Chen Y, et al. Tailoring capsid-directed evolution technology for improved AAV-mediated CAR-T generation. Mol Ther. 2025 Jun 4;33(6):2801-2818. doi: 10.1016/j.ymthe.2024.12.012. Epub 2024 Dec 12. PMID: 39673125; PMCID: PMC12172172.
- Song F, Tsahouridis O, Stucchi S, et al. A multi-kinase inhibitor screen identifies inhibitors preserving stem-cell-like chimeric antigen receptor T cells. Nat Immunol. 2025 Feb;26(2):279-293. doi: 10.1038/s41590-024-02042-1. Epub 2025 Jan 8. PMID: 39779871; PMCID: PMC11785528.

