Uncategorized Thursday, 2025/11/13
The paper introduces a new tool called LOCL-TL for precise studies of local translation, with new findings on two classes of proteins involved in mitochondrial local translation.
Human cells produce various proteins, each with specific functions, often requiring them to be located at specific sites in the cell where that function is needed. One way cells ensure that certain proteins reach the correct location at the right time is through a process called local translation, which ensures proteins are synthesized near where they are needed.
Jonathan Weissman, a member of the Whitehead Institute, and his colleagues have previously studied local translation to understand how it affects cell function and enables cells to respond quickly to changing conditions.
Now, Weissman and his postdoc, Jingchuan Luo, have expanded the understanding of mitochondrial local translation. Near mitochondria, the energy generators of the cell, they published a paper in the journal Cell, introducing a new tool named LOCL-TL for precise studies of local translation, along with new findings on two classes of proteins involved in mitochondrial local translation.
The significance of mitochondrial local translation relates to its unique origin. Mitochondria used to be bacteria that lived inside our ancestral cells. Over time, these bacteria lost their autonomy and became integral parts of the larger cell, including transferring most of their genes to the cell’s nuclear genome.
Cells have evolved processes to ensure that proteins required by mitochondria, encoded by the larger cell’s genome, are transported to the mitochondria. Mitochondria retain a small number of genes in their own genome, so coordination between protein production from mitochondrial and nuclear genomes is needed to avoid mismatched production of mitochondrial components. Local translation may help manage interactions between mitochondrial and nuclear protein production, among other purposes.
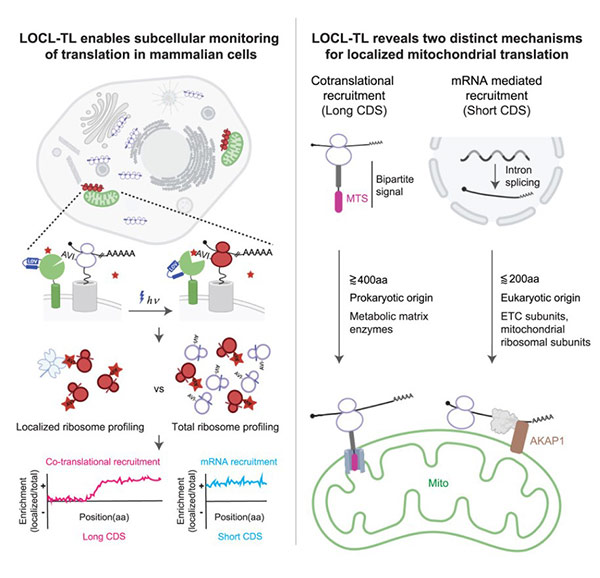
Fig1. Graphical abstract
How to detect local protein production
Protein synthesis requires reading the genetic code stored in DNA into RNA, which is then read or translated by ribosomes, cellular machines that construct proteins according to the RNA code. The Weissman lab previously developed a way to study local translation by tagging ribosomes near structures of interest, capturing these working tagged ribosomes, and observing the proteins they are making.
This approach, called proximity-specific ribosome profiling, allows researchers to see which proteins are being made, and where, within the cell. The challenge Luo faced was adjusting this method to capture only the ribosomes working near mitochondria.
Ribosomes work quickly, and ribosomes tagged while making proteins near mitochondria could move to other parts of the cell to make other proteins within minutes. The only way researchers could ensure that the captured ribosomes were still making proteins near mitochondria was by conducting the experiment very rapidly.
Weissman and colleagues previously addressed this time sensitivity in yeast cells using a ribosome-tagging tool called BirA that is activated by a molecule called biotin. BirA is fused to the structure of interest and tags accessible ribosomes, but only after activation. The researchers limited tagging time by keeping cells deficient in biotin until they were ready to capture the ribosomes. However, the technique is unsuitable for mitochondria in mammalian cells, which need biotin to function normally, so they cannot be made biotin-deficient.
Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| birA-339E |
Recombinant E.coli birA, His-MBP tagged

|
E.coli | E.coli | His | 1-321 a.a. | |
| BIRA-0781B | Recombinant Bacillus subtilis BIRA protein, His-tagged | E.Coli/Yeast | Bacillus subtilis | His |
|
|
| MRRF-1045H | Recombinant Human MRRF, GST-tagged | E.coli | Human | GST | 1-262aa | |
| MRRF-3802H | Recombinant Human MRRF protein, His-tagged | E.coli | Human | His | 1-262 aa | |
| RRS1-2448H | Recombinant Human RRS1, GST-tagged | E.coli | Human | GST | 1-365aa | |
| RPF1-14395M | Recombinant Mouse RPF1 Protein | Mammalian Cells | Mouse | His |
|
|
| RPF1-384Z | Recombinant Zebrafish RPF1 | Mammalian Cells | Zebrafish | His |
|
|
| RBFA-663H | Recombinant Human RBFA Protein, His-tagged | E.coli | Human | His |
|
|
| AKAP1-9517H | Recombinant Human AKAP1 protein, His-tagged | E.coli | fruit fly | His | 31-351 aa | |
| AKAP1-1469M | Recombinant Mouse AKAP1 Protein | Mammalian Cells | Mouse | His |
|
|
| AKAP1-286R | Recombinant Rhesus monkey AKAP1 Protein, His-tagged | Mammalian Cells | Rhesus macaque | His |
|
Luo and Weissman adapted existing tools to respond to blue light instead of biotin. The new tool, LOV-BirA, is fused to the mitochondrial outer membrane. Cells are kept in darkness until the researchers are ready, then exposed to blue light, activating LOV-BirA to tag ribosomes. The researchers wait a few minutes, then quickly extract the ribosomes, and this method proved highly accurate in capturing ribosomes working specifically at mitochondria.
The researchers then used an original method from the Weissman lab for extracting fragments of RNA inside ribosomes. This allows them to see the stage of protein production at which the ribosome was captured, revealing if the entire protein is made at mitochondria or completed elsewhere.
“One advantage of our tool is its granularity,” said Luo. “Being able to see which parts of proteins are locally translated helps us learn more about how local translation is regulated, allowing us to understand its dysregulation in diseases and potentially control local translation in future studies.”
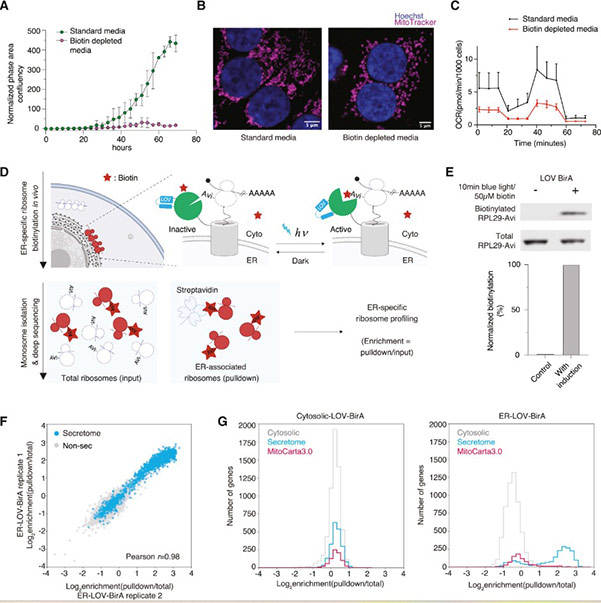
Fig2. Optogenetically controlled proximity-specific ribosome profiling (LOCL-TL)
Two groups of proteins made in mitochondria
Using these methods, the researchers found that about 20% of the genes needed by mitochondria, but located in the main cellular genome, are translated locally at the mitochondria. These proteins fall into two distinct groups with different evolutionary histories and local translation mechanisms.
One group consists of relatively large proteins, each with over 400 amino acids or protein building blocks. These proteins tend to have bacterial origins (present in ancestral mitochondria) and are locally translated in both mammalian and yeast cells, indicating their local translation has been preserved throughout evolutionary history.
Like many mitochondrial proteins encoded in the nuclear genome, these proteins contain a mitochondrial targeting sequence (MTS), a “postal code” indicating where they should be sent. The researchers found that most MTS-containing proteins also have an inhibitory sequence nearby that halts their import until they are fully made. However, this group of locally translated proteins lacks the inhibitory sequence, allowing them to be imported into mitochondria while being produced.
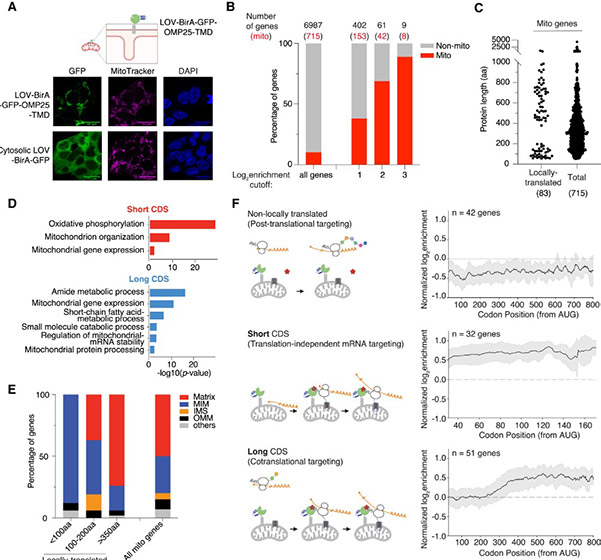
Fig3. Mitochondrial-specific LOCL-TL reveals two distinct classes of mitochondrially localized translation
These longer proteins begin synthesis anywhere in the cell, and after approximately the first 250 amino acids are made, they are transported to mitochondria. As the remaining protein is made, it is simultaneously fed into a channel leading inside mitochondria, occupying it for extended periods and limiting other proteins’ input, so the cell can only afford such synchronized production and import for select proteins.
The researchers hypothesize that these bacterial-origin proteins are afforded priority as an ancient mechanism to ensure accurate production and placement inside mitochondria.
The second group of locally translated proteins is composed of short proteins, each under 200 amino acids long. These proteins evolved more recently, and accordingly, their local translation mechanism is not shared with yeast. Their mitochondrial recruitment occurs at the RNA level. Each RNA molecule’s regulatory region contains two sequences not encoding the final protein but instead encoding cellular machinery to recruit the RNA to mitochondria.
The researchers sought molecules likely involved in this recruitment process and identified AKAP1 , an RNA-binding protein located in the mitochondria. When AKAP1 is removed, short proteins are translated randomly throughout the cell. This provided an opportunity to learn more about the impact of local translation by observing what happens when it is impaired. When short proteins are not locally translated, it leads to the loss of various mitochondrial proteins, including those involved in oxidative phosphorylation, the main energy-generating pathway in our cells.
In future research, Weissman and Luo plan to further explore how local translation affects mitochondrial function and dysfunction in diseases. They also intend to use LOCL-TL to investigate local translation in other cellular processes, such as those related to embryonic development, neuronal plasticity, and disease.
“This approach should be widely applicable to different cellular structures and types, providing many opportunities to understand how local translation facilitates biological processes,” Weissman said. “We’re particularly interested in learning about the roles it may play in diseases, such as neurodegenerative disorders, cardiovascular diseases, and cancer.”
Related Products & Services
- Avi-tagged Proteins
- Fluorescent-labeled Recombinant Proteins
- Site-specific Labeled Recombinant Proteins
- Heavy-labeled Full-length Proteins
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Jingchuan Luo et al, Proximity-specific ribosome profiling reveals the logic of localized mitochondrial translation, Cell (2025). DOI: 10.1016/j.cell.2025.08.002.
