Uncategorized Monday, 2025/11/24
The story of ADAR1 is a perfect example of how basic biological research can reveal the fundamental laws of life and ultimately bring revolutionary breakthroughs in the treatment of major diseases.
In every cell of our body, a silent “hidden battle” is constantly taking place - the RNA in the cell sometimes forms double-stranded RNA (dsRNA) structures similar to viral RNA. If they are mistakenly judged by the immune system as “foreign invasion”, it will trigger a terrible autoimmune storm. The key to preventing this disaster is a protein called ADAR1.
However, ADAR1 has a dual nature. It not only plays a role in maintaining immune balance in the body to prevent autoimmune diseases, but also promotes cancer progression, immune evasion, and treatment resistance.
Recently, the review journal Trends in Cell Biology under Cell Press published a review paper titled "ADAR1: from basic mechanisms to inhibitors" from the University of Oxford. This review systematically combs through the latest research progress of ADAR1, interprets its dual nature - the absence of ADAR1 will lead to autoimmune diseases, while its expression will promote the development and metastasis of cancer. The review also explores the significance of ADAR1 for human diseases - developing ADAR1 inhibitors for cancer treatment.
Our Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| ADAR-510R | Recombinant Rat ADAR Protein | Mammalian Cells | Rat | His |
|
|
| ADAR-528H | Recombinant Human ADAR protein, MYC/DDK-tagged | HEK293 | Human | DDK&Myc | 1-1226 aa |
|
| ADAR-529H | Recombinant Human ADAR protein, GST-tagged | Wheat Germ | Human | GST | 2-110 a.a. |
|
| ADAR-9137Z | Recombinant Zebrafish ADAR | Mammalian Cells | Zebrafish | His |
|
|
| ADAR-01H | Recombinant Human ADAR Protein, DYKDDDDK-tagged | Human Cells | Human | Flag |
|
|
| IFIH1-1143H | Recombinant Human IFIH1 Protein, His (Fc)-Avi-tagged | HEK293 | Human | Avi&Fc&His | ||
| IFIH1-1252H |
Recombinant Human IFIH1 Protein, His-tagged

|
E.coli | Human | His | 700-1025 a.a. |
|
| IFIH1-2201R | Recombinant Rhesus monkey IFIH1 Protein, His-tagged | Mammalian Cells | Rhesus macaque | His |
|
|
| IFIH1-28633TH | Recombinant Human IFIH1 | Wheat Germ | Human | Non | 928-1023 a.a. |
|
| PRKRA-1960H | Recombinant Human PRKRA, GST-tagged | E.coli | Human | GST | 1-313aa | |
| PRKRA-1175HFL | Recombinant Full Length Human PRKRA Protein, C-Flag-tagged | Mammalian Cells | Human | Flag | Full L. |
|
| PRKRA-1769H | Recombinant Human PRKRA Protein, His (Fc)-Avi-tagged | HEK293 | Human | Avi&Fc&His |
|
|
| EIF2A-12348H | Recombinant Human EIF2A, GST-tagged | E.coli | Human | GST | C-term-350a.a. | |
| EIF2A-30H |
Active Recombinant Human EIF2A
|
Sf9 Cells | Human | Non |
|
|
| EIF2A-18H | Recombinant Human EIF2A protein, His-tagged | E.coli | Human | His |
|
|
| ZBP1-3779H | Recombinant Human ZBP1, GST-tagged | E.coli | Human | GST | 1-149aa | |
| ZBP1-897H | Recombinant Human ZBP1 protein, His-tagged | E.coli | Human | His | Met1-Ser149 |
|
ADAR1: The "Self-Identification" Engineer of Cells
The core function of ADAR1 is "RNA editing". As an RNA editing enzyme, it can convert adenosine (A) into inosine (I) in double-stranded RNA (dsRNA) molecules, a process known as A-to-I editing. Inosine (I) is "read" as guanosine (G) in the cell. Therefore, A-to-I editing is recognized as A-to-G, thus achieving gene editing at the RNA level. More importantly, A-to-I editing changes the structure of dsRNA, making it "irregular". This structural change is equivalent to putting a “friendly” label on the self-RNA, thus avoiding being mistakenly attacked by the immune system.
ADAR1 mainly has two subtypes - ADAR1p110: It is stationed in the cell nucleus for a long time, mainly responsible for basic RNA editing, which is related to cell development and homeostasis maintenance; ADAR1p150: It is produced by interferon induction and is a “star player”. It has a unique structure that can shuttle between the cell nucleus and the cytoplasm, especially its Za domain can recognize an unusual Z-RNA. ADAR1p150 is the key defense against excessive activation of the immune system.
Once the Defense is Breached: ADAR1 Deficiency and Autoimmune Storm
If the function of ADAR1 (especially the p150 subtype) is abnormal, the consequences are catastrophic. Studies have shown that the absence or mutation of ADAR1 in cells will lead to severe autoimmune inflammatory diseases in both humans and mice.
When ADAR1 is absent, the “native” endogenous dsRNA that has not been edited will be exposed, activating multiple “alarms” in the cell - dsRNA sensors:
MDA5 : Like a sentry, it can recognize long, perfect dsRNA. Once activated, it will start interferon expression through the MAVS protein signaling pathway, triggering a systemic alarm;
PKR : After being activated, it will phosphorylate the eIF2α protein, pressing the “pause button” for global protein synthesis, starting the integrated stress response, and may eventually lead to cell death;
ZBP1 : A particularly interesting sensor, it can recognize unusual Z-RNA. The Za domain of ADAR1p150 can also bind to Z-RNA, thus competing with ZBP1 and inhibiting its activation. Once ZBP1 is activated, it will trigger programmed cell death and inflammation.
In humans, the absence of ADAR1 will manifest as severe brain diseases such as Aicardi-Goutières syndrome (AGS), in which patients continuously produce interferon, as if they were in a state of viral infection. In mice, complete knockout of ADAR1 will directly lead to embryonic lethality.
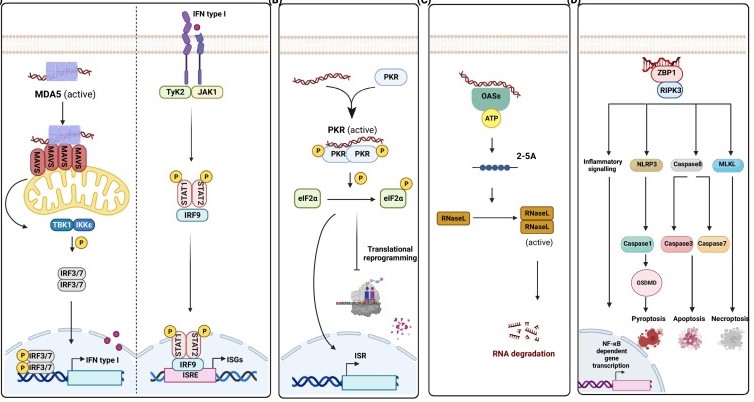
Fig1. In the absence of adenosine deaminase acting on RNA 1 (ADAR1) or its editing activity, double-stranded RNA (dsRNA) activates various dsRNA sensors.
Changing from Defense to Offense: The “Dual Role” of ADAR1 in Cancer
Interestingly, this “guardian” that maintains the homeostasis of the immune system may be “subverted” by cancer cells.
In some cancers, ADAR1 plays the role of an “accomplice”. Cancer cells have unstable genomes and produce a large amount of endogenous dsRNA. To avoid being cleared by the immune system, cancer cells upregulate the expression of ADAR1 and use its function to “disguise” themselves, thus escaping immune surveillance.
This also provides a brilliant idea for cancer treatment - if the function of ADAR1 is inhibited, wouldn't it be like tearing off the “disguise” of cancer cells, allowing the immune system to recognize and attack them?
This is one of the most exciting directions in the current field of cancer immunotherapy - “viral mimicry”. By inhibiting ADAR1, cancer cells accumulate a large amount of unedited dsRNA, simulating the state of viral infection, activating the innate and adaptive immune responses in the body, and thus killing tumors.
Many studies have shown that the combination of ADAR1 inhibitors with immune checkpoint blockade therapy (such as anti-PD-1 monoclonal antibodies) or epigenetic drugs (such as DNMT inhibitors) can produce significant synergistic effects and greatly enhance the anticancer effect.
The Future is Promising: Development Strategies for ADAR1 Inhibitors
Based on such a bright application prospect, the development of ADAR1 inhibitors has become a new hotspot. The review introduces six different strategies to inhibit the pro-tumor effects of ADAR1 –
Accumulate dsRNA : Using DNA methyltransferase inhibitors (DNMTi), splicing inhibitors, or XRN1 inhibitors will increase the level of endogenous immunogenic dsRNA, which may saturate the RNA editing capacity of ADAR1, thus allowing the immune system to sense dsRNA;
Inhibit the binding of ADAR1 to dsRNA;
Inhibit the deaminase activity of ADAR1;
Inhibit the protein-protein interactions of ADAR1 with downstream sensors.
These three methods may all lead to the accumulation of unedited dsRNA, which can be perceived by the immune system;
Deplete ADAR1, by using PROTAC to degrade ADAR1 protein, or regulating the processing of ADAR1 precursor mRNA to specifically reduce the expression of the ADAR1p150 subtype, which can also achieve the accumulation of unedited dsRNA;
“Bypass” activation, directly activate downstream sensors of ADAR1 such as ZBP1, to simulate the effect after ADAR1 is inhibited.
Summary and Outlook
The story of ADAR1 is a perfect example of how basic biological research can reveal the fundamental laws of life and ultimately bring revolutionary breakthroughs in the treatment of major diseases. From preventing autoimmune diseases to fighting cancer, research in the field of ADAR1 is developing rapidly.
In the future, researchers still need to answer several key questions:
Which specific endogenous dsRNA is most likely to trigger autoimmune diseases?
How to balance the therapeutic effect and potential autoimmune risk when inhibiting ADAR1?
Can high-selectivity inhibitors that only target the ADAR1p150 subtype in cancer cells be developed?
The ADAR (Adenosine deaminases acting on RNA) family is the key enzyme that catalyzes RNA A-to-I editing. It plays a core role in regulating RNA diversity and maintaining immune homeostasis and nervous system function. In recent years, RNA editing technology based on ADAR has developed rapidly. Compared with the CISRPR gene editing technology, it does not require the introduction of exogenous editing enzymes or effector proteins, avoiding delivery difficulties and related immunogenicity issues. In addition, it does not change DNA and is safer. It has great potential in the treatment of genetic diseases and is being developed as a new generation of gene editing therapies.
In addition, many studies have shown that ADAR1 is not only an RNA editing enzyme, but also closely related to autoimmune diseases and cancer, and has become an important new target for disease treatment.
Related Products & Services
- Immune Checkpoint Proteins
- ADC Target Protein
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- ADAR1: from basic mechanisms to inhibitors. Rehwinkel, Jan et al. Trends in Cell Biology, Volume 35, Issue 1, 59 - 73
