Uncategorized Thursday, 2025/11/13
The Human Body's Natural "Cancer Brake" Has Been Found!
In images achieved at single-atom resolution, researchers demonstrated how this "brake" functions: a protein binds to a cancer-related segment of DNA, much like two Lego blocks snapping together.
Is it possible to harness the natural cancer inhibition mechanism to stop this disease? It might be, but to harness the systems designed by nature, we first need to understand it. Researchers, led by a team from Purdue University, are exploring a molecular mechanism that can suppress the rampant cell division associated with cancer. Their work opens the door to developing drugs that could exploit this mechanism.
"We think that this system was naturally evolved in the context of cancer. It's like a braking system. So, the question we're now asking is, 'How can we harness it for drug discovery?'" stated Danzhou Yang, Martha and Fred Borch Chair in Cancer Therapeutics at Purdue’s College of Pharmacy and Distinguished Professor in the Department of Medicinal Chemistry and Molecular Pharmacology. The team's latest study is published in the journal Science.
In images achieved at single-atom resolution, researchers showed how this "brake" functions: a protein binds to a segment of DNA related to cancer much like two Lego blocks fitting together. This segment of DNA is part of the c-MYC gene, which, when aberrantly regulated, is a primary driver of all blood cancers and 80% of human solid tumors.
The MYC oncogene plays a significant role in cancer initiation and progression; it is the "master regulator" driving cell growth, proliferation, and metabolism. When the c-MYC gene combines with the nucleolin protein, the gene cannot replicate, thus blocking a crucial step in uncontrolled cell division.
Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| MYC-1029H | Recombinant Full Length Human MYC, His-tagged | E.coli | Human | His | Full L. 1-454 a.a. | |
| MYC-1028H |
Active Recombinant Human MYC
|
Sf9 Cells | Human | Non |
|
|
| MYC-2522H |
Active Recombinant Human MYC, His-tagged
|
E.coli | Human | His |
|
|
| MYC-79H |
Active Recombinant Human MYC protein, arginine-tagged
|
E.coli | Human |
|
||
| MYC-152H | Recombinant Human MYC tag protein | E.coli | Human | Non |
|
|
| MYC-254H | Recombinant Human MYC protein, T7/His-tagged | E.coli | Human | His&T7 |
|
|
| MYC-2641C | Recombinant Chicken MYC | Mammalian Cells | Chicken | His |
|
|
| MYC-26H | Recombinant Human MYC protein, MYC/DDK-tagged | HEK293 | Human | DDK&Myc |
|
|
| NCL-1493M | Recombinant Mouse NCL Protein, His-tagged | E.coli | Mouse | His | 353-568 a.a. | |
| NCL-05H | Recombinant Human NCL protein, His-tagged | Yeast | Human | His |
|
|
| NCL-06H | Recombinant Human NCL protein, His-tagged | E.coli | Human | His | 388-569 a.a. |
|
Determining the structure of the complex formed between the DNA and this protein is an achievement itself, but the unusual shape of this complex is equally enlightening. Yang's team found that the nucleolin 's four modules, like pearls on a string, bind to the four linked rings of DNA with a novel globular structure.
"What we found completely changed the paradigm. No one anticipated this binding mode of DNA," Yang said. "Each globule binds weakly to one ring. But when combined, you have this synergistic binding that is very specific and very strong."
DNA encodes instructions for building proteins using just four types of nucleotide molecules, similar to Morse code. When not in use, the two DNA strands in a cell's genome bind together like a zipper, forming the familiar double-helix structure. However, throughout the cell's lifecycle, the strands periodically "unzip" along their length, allowing genes on one strand to be transcribed into RNA — the first step in gene expression for producing materials as per DNA instructions.
Some genes encode instructions for materials that help a cell divide into two, a tightly controlled intricate process. Mutations, environmental factors, and changes in the frequency of specific gene expression can disrupt this process, leading to unchecked cell growth and division, thus causing cancer.
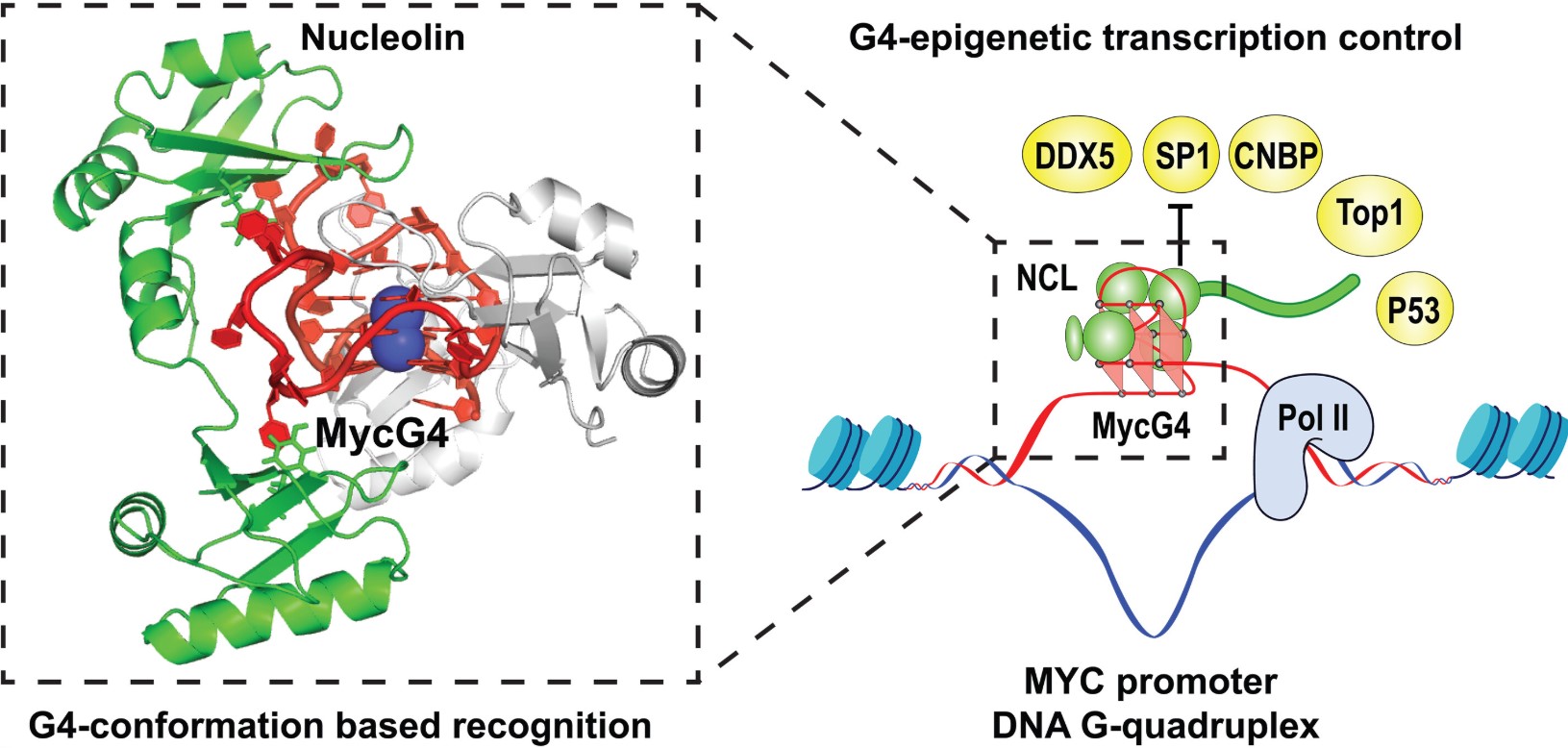
Fig1. G4 conformation–based protein recognition for G4 epigenetic transcription control.
The c-MYC gene is part of this tight regulation, encoding instructions to produce the c-MYC protein, a member of the transcription factor family, which acts like an on-switch for many other genes by controlling the amounts of proteins they express. c-MYC helps regulate cell processes such as growth, division, and even immune responses. Mutant forms of the c-MYC gene drive cancer by producing excess amounts of the c-MYC transcription factor, disrupting the proper pace of transcription.
However, according to Yang, somehow evolution has recognized this issue and taken containment measures. When DNA unzips, fragments of DNA rich in guanine nucleotides sometimes gather together to form an odd temporary “knot” known as a G-quadruplex. Such regions are found in the c-MYC gene, and the frequency of G-quadruplex "knot" formation is much higher than in healthy cells when the c-MYC gene is accessed with the aberrant rapidity consistent with cancer.
But if nucleolin binds to the G-quadruplex, it stabilizes this "knot" and inhibits the transcription machinery from connecting with the gene, thus aborting transcription. This deceleration is only temporary since the G-quadruplex is short-lived. However, it serves as an effective mechanism for halting cancer — a mechanism researchers can potentially enhance. "If we can target this G-quadruplex and stabilize its complex with nucleolin, extending its lifetime significantly, we can shut down the transcription of c-MYC. This could suppress or even cure cancer," Yang noted.
c-MYC is infamously known for its role in promoting cancer, and Yang says it is a hot target for drug discovery. The c-MYC G-quadruplex is a viable drug target, and understanding the precise three-dimensional structure of the G-quadruplex-nucleolin complex at the atomic level — how these Lego blocks snap together — makes it possible to design a drug that adheres to both the G-quadruplex and nucleolin, but for a longer duration.
This new study provides such detailed information and unveils the remarkable feature of the four-part binding between the modular protein and the globular DNA rings. Their detailed structural diagram shows how the globular G-quadruplex DNA presents four rings offering docking sites for the protein. Typically, modular proteins like nucleolin form only weak connections with DNA, but in this case, nucleolin’s four modules bind to all four rings on the globular DNA.
"We can see how this modular protein engages all four rings of the globular DNA G-quadruplex structure, forming a very strong binding," Yang commented. "This is significant not only for cancer but for various other aspects of biology. G-quadruplexes can form in many guanine-rich sequences with critical functional roles."
In further research, Yang intends to continue gathering details on how G-quadruplexes interact with other proteins to control transcription, which Yang refers to as "G-quadruplex-based epigenetic transcription control," thereby sketching a clearer blueprint for drug discovery. With this information, her team will be exploring drugs that can bind to the G-quadruplex and its associated proteins, seeking what Yang calls "epigenetic transcription control inhibitors" that could combat cancer.
Related Products & Services
- Cytokines for Organoid Culture
- ADC Target Protein
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Immune Checkpoint Proteins
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Luying Chen et al, Structural basis for nucleolin recognition of MYC promoter G-quadruplex , Science (2025). DOI: 10.1126/science.adr1752.
