Uncategorized Sunday, 2025/11/23
Researchers have developed a powerful upgraded therapy: an engineered antibody that achieves the therapeutic efficacy of intravenous immunoglobulin (IVIG) in mice at extremely low doses, and can be synthesized without human plasma.
Intravenous immunoglobulin (IVIG) therapy treats autoimmune diseases by infusing naturally occurring IgG antibodies into patients. Dating back to the 1950s, this therapy is currently FDA-approved for only four diseases but is widely used off-label for over 80 other conditions, as it is often the only effective treatment available.
However, IVIG has significant drawbacks. Treatment may require multiple, hours-long high-dose infusions each month, at a high cost, and supply shortages are common due to the reliance on donated human plasma.
Now, scientists at the Leonard Wagner Laboratory of Molecular Genetics and Immunology at Rockefeller University have leveraged a previously unknown mechanism in an anti-inflammatory pathway to develop a powerful upgraded therapy: an engineered antibody that achieves IVIG's efficacy in mice at very low doses, without the need for human plasma. The study was published in the journal Science.
Andrew Jones, the first author of the study and a research assistant in the lab led by Jeffrey Ravetch, said, "We found that by enhancing the binding of a particular receptor pair, we can significantly reduce the dose while achieving the same effect."
These advances build on the lab's earlier work, which had already developed a molecule ten times more potent than IVIG, currently in Phase 2 clinical trials through the biotech company Nuvig co-founded by Ravetch. The current findings greatly improve upon that molecule.
Based on 40 years of research on Fc receptors
These discoveries are based on 40 years of research on Fc receptors by the Ravetch lab. Fc receptors are a family of proteins present on the surface of almost all immune cells, to which antibodies bind to coordinate the effector responses of the immune system. The most common serum antibody is immunoglobulin G, which accounts for 75% of the antibodies in the blood that fight infections—and is a key component of IVIG.
Research into the anti-inflammatory properties of IVIG began about 25 years ago when Ravetch discovered that a small fraction of serum IgG in IVIG had a naturally occurring modification: a sialylated sugar modification that endowed it with anti-inflammatory properties.
Subsequent studies in his lab identified two additional components required to trigger IVIG's anti-inflammatory response: an inhibitory Fc receptor called FcγRIIB and a lectin called DC-SIGN. These findings enabled them to develop the drug now in Phase 2 clinical trials, NVG-2089, which is ten times more potent than IVIG in suppressing autoimmune inflammation.
"These are the parts we've figured out," Ravetch said. "The question is, how do these three components work together to mediate anti-inflammatory activity? That's what our current research is all about."
The early work was also done using mice with their own natural Fc receptors (not human Fc receptors) to study IVIG activity. Since then, Ravetch has bred mice that express human Fc receptors.
Jones said, "We thought that if we could better understand how IVIG specifically acts on cells expressing human Fc receptors, we might be able to develop the next generation of therapeutic IVIG."
Related Proteins
Potency Upgrade
To understand how these components work together to mediate IVIG activity, the researchers conducted numerous in vitro experiments, testing various activation and interaction scenarios.
"We found that the type I FcγRIIB receptor and the type II DC-SIGN accessory receptor actually bind to each other on the cell surface, which seems to be important for the anti-inflammatory effect of IgG," Jones said. "This is a novel configuration we've never seen before. We think that when they bind, it enhances the ability of sialylated IgG antibodies to trigger anti-inflammatory signaling cascades."
Next, they designed a recombinantly expressed IgG to enhance its binding to these receptors and infused it into mice with induced arthritis (meaning they were injected with serum isolated from mice with naturally occurring arthritis) that expressed human Fc receptors. Another group of similar arthritic mice was treated with traditional IVIG infusions.
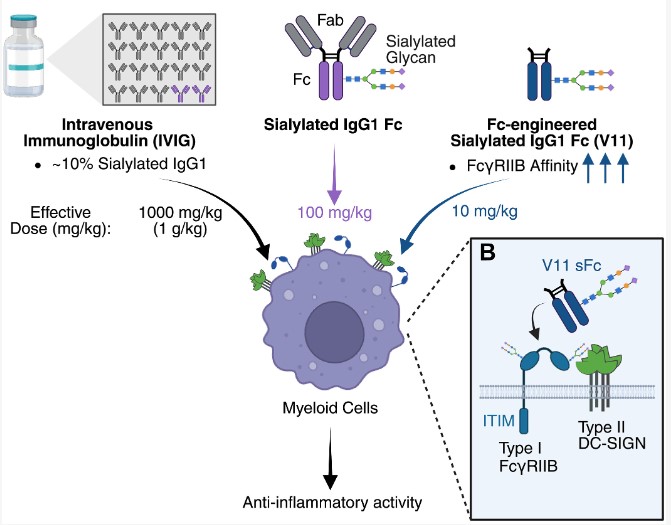
Both groups of mice benefited from the infusions, with reduced joint swelling. But the dose difference was enormous: to achieve the same effect as a single dose of the new molecule, 100 times the dose of IVIG was required.
"This is a very significant difference, and its importance is reflected in several ways. First, this new molecule is a recombinant protein that we can produce in vitro, so it doesn't require human plasma. That's a huge advantage," Ravetch said. "Second, there are currently many autoimmune diseases that are not treated with IVIG because we can't achieve the right dosing. With this highly potent product, it may be possible to achieve the right dose and expand its application to more autoimmune diseases."
In a second test, a mouse model used as a substitute for multiple sclerosis, an autoimmune disease that leads to worsening cognitive and motor abilities, was employed. The molecule protected the mice from neuroinflammation at the same low dose by blocking cell destruction.
Looking ahead, the lab will study the structure and molecular dynamics of the type I and type II receptors. They have identified many over the years, but how they pair and what their functions are remains to be discovered.
Jones said, "Our findings open the door to exploring how they function in different biological pathways."
They will also explore its clinical potential. Ravetch said, "So far, we have licensed the molecule to Nuvig, who will conduct further testing to decide whether to develop it as a clinical product. We hope to see it applied to patients."
Related Products & Services
- Fc Receptors
- Immune Checkpoint Proteins
- ADC Target Protein
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Andrew T. Jones et al, The anti-inflammatory activity of IgG is enhanced by co-engagement of type I and II Fc receptors, Science (2025). DOI: 10.1126/science.adv2927.

