Uncategorized Thursday, 2025/11/13
This study found in C57BL/6J mice that male dietary macronutrients affect over 50% of the brown adipose tissue (BAT) proteome and negatively regulate the expression of basement membrane proteoglycan in daughters’ BAT, providing a basis for dietary intervention in metabolic health.
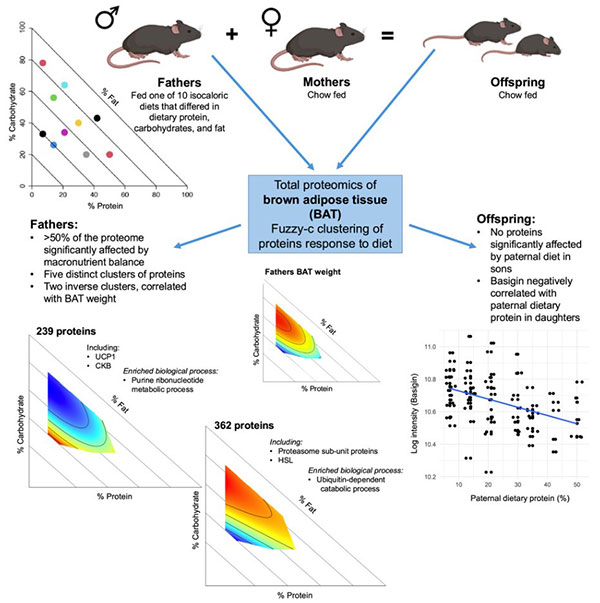
Fig1. Graphical abstract
Modern individuals increasingly focus on the relationship between diet and health, from weight management to the prevention of metabolic diseases, with dietary details closely linked to bodily functions. Brown adipose tissue (BAT), as a key organ in regulating energy expenditure and maintaining metabolic homeostasis, is a focal point in the study of how diet influences this process and whether it is transmitted to offspring through paternal lineage.
Recently, a study published in Cell Reports titled “Dietary macronutrients modulate the proteome of brown adipose tissue in males and their female offspring” systematically explored how male dietary macronutrients (proteins, carbohydrates, fats) regulate the BAT proteome in both themselves and their female offspring.
The study involved male C57BL/6J mice (F0 generation, fathers) who were provided with ten diets with consistent caloric content but varying macronutrient proportions. Results showed that F0 males on a low-fat diet (particularly moderate protein, high carbohydrate combination) experienced the highest weight gain and significant increases in both subcutaneous white adipose tissue (WAT) and interscapular BAT weight. On a low-protein diet, increased food intake demonstrated the protein leverage effect.
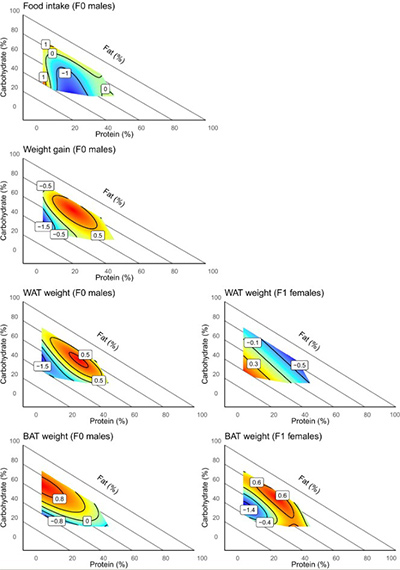
Fig2. Surface plots of F0 male food intake, F0 male weight gain, F0 male WAT weight, F0 male BAT weight, as well as F1 female offspring WAT weight and F1 female offspring BAT weight across the 10 diets
Analysis of the F0 males’ BAT proteome identified 2,582 proteins, with 1,385 significantly affected by diet and classified into five protein clusters with different nutritional response patterns. Clusters 3 and 4 exhibited opposite expression patterns and both were related to BAT weight: Cluster 3 proteins (including uncoupling protein 1 UCP1 and creatine kinase BCKB ) showed high expression under high-fat diets (low carb, low protein), enriched in purine synthesis and metabolism; Cluster 4 proteins (including proteasomal subunits PSMB2, PSMC5, PSMD4, and valosin-containing protein VCP ) showed high expression under low-fat diets, involved in ubiquitin-dependent protein degradation. The expression trend of Cluster 4 proteins corresponded with BAT weight in F0 males, while Cluster 3 showed an opposite correlation—UCP1, as a key protein in BAT non-shivering thermogenesis, showed expression negatively correlated with BAT weight. High BAT weight under low-fat diets but low UCP1 expression suggested non-UCP1 dependent thermogenic mechanisms. Additionally, the high expression of proteasomal proteins in Cluster 4 under low-fat diets might maintain protein homeostasis under cellular stress in BAT, while changes in purine synthesis-related proteins in Cluster 3 might affect UCP1 activity by modulating its inhibitors (purine compounds).
Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| UCP1-27R | Recombinant Rat UCP1 protein, His-tagged | E.coli | Rat | His | 179-296 a.a. | |
| UCP1-28R | Recombinant Rat UCP1 protein, His/GST-tagged | E.coli | Rat | GST&His | 179-296 a.a. | |
| UCP1-9176H | Recombinant Human UCP1, His-tagged | E.coli | Human | His | 96-185a.a. | |
| Ucp1-281M | Recombinant Mouse Ucp1 Protein, His-tagged | E.coli | Mouse | His | Pro179~Leu296 | |
| Ucp1-283R | Recombinant Rat Ucp1 Protein, His/GST-tagged | E.coli | Rat | GST&His | Pro179~Leu296 | |
| UCP1-2916H | Recombinant Human UCP1 protein, His-tagged | E.coli | Human | His | 1-307 aa | |
| CKB-11263H | Recombinant Human CKB, GST-tagged | E.coli | Human | GST | 1-381a.a. | |
| CKB-2729H | Recombinant Human Creatine Kinase, Brain, His-tagged | E.coli | Human | His | ||
| CKB-495H |
Recombinant Human CKB protein, His-tagged

|
Insect Cells | Human | His | 2-281 a.a. |
|
| PSMB2-1321H | Recombinant Human Proteasome (prosome, macropain) Subunit, Beta Type, 2, His-tagged | E.coli | Human | His |
|
|
| PSMB2-30366TH | Recombinant Human PSMB2, His-tagged | E.coli | Human | His | Full L. 1-201 a.a. |
|
| PSMC5-3184H | Recombinant Human PSMC5 protein, His-tagged | E.coli | Human | His | 1-406 aa | |
| PSMC5-1080Z | Recombinant Zebrafish PSMC5 | Mammalian Cells | Zebrafish | His |
|
|
| PSMD4-2031H | Recombinant Human PSMD4, His-tagged | E.coli | Human | His | 1-377aa | |
| PSMD4-1810H | Recombinant Human PSMD4 protein, GST-tagged | E.coli | Human | GST | 1-377 aa | |
| PSMD4-422H | Recombinant Human PSMD4 Protein, His/GST-tagged | E.coli | Human | GST&His | Met1~Lys377 | |
| VCP-3656H | Recombinant Human VCP protein, GST-tagged | E.coli | Human | GST | 345-644 aa |
In terms of transgenerational effects, analysis of F1 female (daughters) and male (sons) offspring BAT proteomes showed that paternal diet only affected the daughters’ BAT proteome: the expression of basement membrane glycoprotein (BSG) in daughters’ BAT negatively correlated with paternal dietary protein proportion—the more protein the father consumed, the lower the BSG expression in the daughters’ BAT. BSG is a known key protein regulating UCP1 transcription and normal BAT function, suggesting that paternal diet might indirectly regulate daughters’ metabolic function by affecting BSG expression in BAT. No detectable effect of paternal diet was found on sons’ BAT proteome, indicating a distinctly gender-specific transgenerational effect.
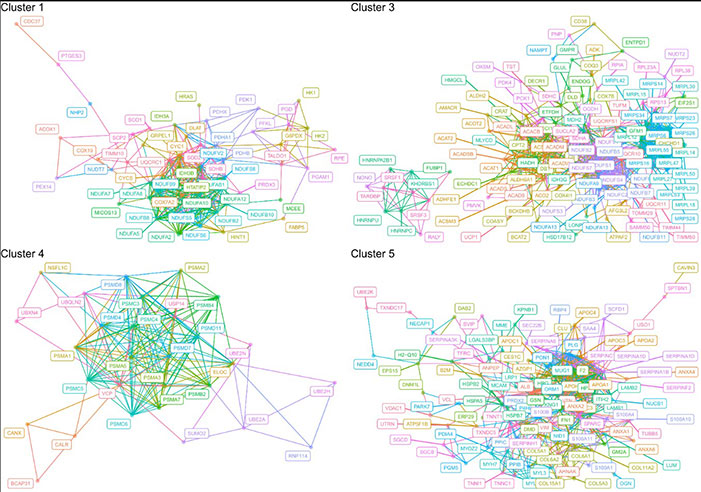
Fig3. Schematic networks of the significant proteins that were involved in enriched biological processes
This study is the first to systematically analyze, at the proteome level, the regulatory effects of male dietary macronutrients on BAT function in themselves and their offspring, revealing specific patterns of dietary influence on BAT protein expression (such as the inverse relationship of UCP1 with BAT weight and the critical role of protein turnover) and discovering specific transgenerational effects on female offspring. These findings provide a new perspective for understanding the relationship between diet and metabolic health, and suggest that optimizing men’s dietary structure might not only improve their own metabolism but also potentially protect their daughters’ metabolic health. This lays the foundation for future exploration of the molecular mechanisms in transgenerational metabolic regulation (such as BSG pathways) and the development of targeted dietary intervention strategies, holding significant theoretical and practical implications for the prevention and management of metabolic diseases.
Related Products & Services
- Obesity Targets
- ADC Target Protein
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Immune Checkpoint Proteins
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Macartney EL, Senior AM, Crean AJ, et al. Dietary macronutrients modulate the proteome of brown adipose tissue in males and their female offspring. Cell Rep. 2025;44(8):116050. doi:10.1016/j.celrep.2025.116050
