Uncategorized Sunday, 2025/09/07
This landmark study offers a new paradigm for understanding leukemia relapse. It elevates a long-neglected cellular by-product—the excised signal circle (ESC)—from a "harmless bystander" to a "central culprit."
In the prolonged battle against cancer, "relapse" is a heart-wrenching term. For pediatric patients with Acute Lymphoblastic Leukemia (ALL), despite modern medicine being able to cure most initial cases, around 15-20% of children still relapse post-treatment. These relapsed leukemia cells are often more aggressive and have developed resistance to chemotherapy. What secrets lie behind this? How do cancer cells, seemingly eradicated, manage to make a comeback?
For a long time, the medical community has been chasing the "ghost" causing relapse. We know that gene mutations are the roots of cancer; however, the pivotal mutations and molecular mechanisms driving relapse largely remain shrouded in mystery. We urgently need to find a "crystal ball" that can predict relapse risks and design more potent treatment protocols tailored for high-risk patients.
On August, a study published in Nature titled "Excised DNA circles from V(D)J recombination promote relapsed leukaemia" unveiled a long-standing "cold case". Researchers focused on a molecule long thought to be harmless "cellular junk"—the circular DNA known as excised signal circles (ESCs). They discovered that these seemingly insignificant DNA remnants play the role of a "ghostly influencer" in specific leukemia cells, silently sowing the seeds of relapse.
This research challenges traditional perceptions of immune system functioning and could fundamentally alter future strategies for diagnosing and treating leukemia.
Restless "Cellular Dust": An Unexpected Legacy of V(D)J Recombination
To understand this story, we must first delve into the artistic biological process within a living organism—V(D)J recombination. Our immune system's ability to recognize and combat thousands of different pathogens lies in the diverse antibodies and T cell receptors (TCRs) produced by B cells and T cells. This process is akin to a skilled genetic "chef" randomly selecting and "cooking" from a vast gene library containing three "ingredients": Variable (V), Diversity (D), and Joining (J). Each assembly creates a unique "recipe": a specific antibody or TCR gene.
In this creative genetic "cooking", unselected gene segments and the intervening DNA are cut out like "trimmings". Instead of being immediately degraded, these "trimmings" self-circularize into a stable closed-loop DNA molecule, today's protagonist—the excised signal circle (ESC).
For decades, classic cell biology textbooks taught us that ESCs are harmless by-products of V(D)J recombination. Lacking a replication origin and a centromere, they can't be evenly distributed to daughter cells during cell division. Theoretically, as cells divide, these ESCs should get "diluted" and ultimately disappear from the cell population, akin to dust blown away by the wind. With this "conventional wisdom", ESCs were deemed functionless "inert molecules".
However, the study authors boldly questioned this "conventional wisdom": are these "cellular dust" truly content to remain silent?
To test this, researchers first conducted analysis on healthy mice's B cells. They devised a clever quantitative PCR method. On ESCs, the recombination signals of V and J gene segments are joined, forming a unique "signal joint" (SJ). Conversely, in the main chromosome, recombined V and J segments form a "coding joint". Researchers precisely measured the ratio of SJ (representing ESCs) to coding joints (representing recombined cells) in B cells at different developmental stages.
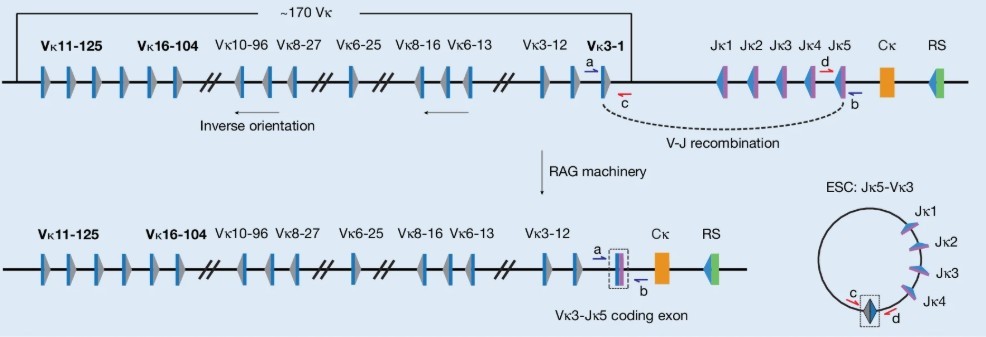
Fig.1 Schematic of the mouse immunoglobulin kappa ( Igk ) locus, highlighting the gene segments studied: Vκ16-104, Vκ3-1 and Vκ11-125, which undergo deletional recombination to generate an ESC.
If conventional wisdom holds, this ratio should significantly decline as pre-B cells develop into mature, multiply divided B cells (IgG+ cells) due to ESC dilution. Surprisingly, experimental results were striking. For various V-J recombinations like Jκ5-Vκ3-1 and Jκ5-Vκ16-104, this ratio not only didn't decline but increased in mature IgG+ cells. This indicates that ESCs aren't being diluted as expected, suggesting some mechanism is preserving them against elimination.
This discovery led to another crucial question: in what form do these surviving ESCs exist? Are they reintegrated into the genome or remain as independent circular entities? To answer this, researchers employed RecBCD nuclease. This "linear DNA killer" can efficiently degrade linear DNA molecules starting from the ends but is powerless against closed-loop DNA without ends.
They extracted high-quality genomic DNA from mature B cells in mouse spleens and treated it with RecBCD. For the linear genome DNA control gene GAPDH, its quantity dropped to nearly undetectable levels post-treatment. However, the SJ sequences representing ESCs retained over 50% post-treatment. This strong evidence suggests that, after several cell divisions, numerous ESCs remain as independent, closed-loop entities persistently existing in mature B cells.
These findings in healthy mice are enough to disrupt conventional understanding. It suggests that ESCs might not be transient "cellular dust" but rather long-term "non-core members" within cells. In diseases characterized by abnormal V(D)J recombination, such as leukemia, what role do these "non-core members" play?
From "Mouse" to "Human": Following the Trail of the Leukemia Relapse Mystery
B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is a malignant tumor originating from B lymphocyte precursors. In this disease, the RAG proteins responsible for V(D)J recombination often get "reactivated" inappropriately. This paints a disturbing picture: a "retired" genetic scissor (RAG protein) resumes work in rapidly proliferating cancer cells, while possibly large amounts of overlooked circular DNA templates (ESCs) float within these cells. Is this the perfect storm recipe?
Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| RAG1-13889M | Recombinant Mouse RAG1 Protein | Mammalian Cells | Mouse | His |
|
|
| RAG1-2853C | Recombinant Chicken RAG1 | Mammalian Cells | Chicken | His |
|
|
| RAG2-2162H | Recombinant Human RAG2, GST-tagged | E.coli | Human | GST | C-term-353aa | |
| RAG2-13890M | Recombinant Mouse RAG2 Protein | Mammalian Cells | Mouse | His |
|
With this in mind, the research team shifted their focus to clinical samples of BCP-ALL. They developed a high-throughput sequencing technique called LAM-ESC (Linear Amplification-Mediated ESC), capable of scanning and quantifying every type of ESC in patient samples. They analyzed bone marrow samples from 71 pediatric BCP-ALL cases at initial diagnosis. Among these children, 34 unfortunately relapsed while the other 37 remained in continuous remission (i.e., did not relapse).
When LAM-ESC analysis results appeared on the computer screen, a shocking scene emerged. Researchers plotted a scatter plot after normalizing each patient's total ESC copies. On the plot, red dots representing relapsed patients and blue dots representing non-relapsed patients formed two distinct groups.
Data shows that among 34 patients in the relapse group, many had significantly higher ESC levels than a specific threshold (set based on the highest ESC level detected in healthy blood samples). In contrast, among 37 patients who did not relapse, most had ESC levels below this threshold, similar to healthy individuals. Through rigorous statistical testing, researchers found a strong correlation between high initial ESC levels and future relapse, with a p-value of 0.00017. It’s akin to saying the initial cellular ESC count can almost predict future relapse akin to a crystal ball.
Of course, rigorous scientific exploration needs to eliminate all possible confounding factors. A natural question arises: Could the relapse group's higher ESC levels simply result from stronger RAG protein activity, producing more ESCs?
To test this possibility, researchers analyzed RAG1 and RAG2 gene expression levels in some patient samples. Surprisingly, they found no direct linear relationship between ESC copy numbers and RAG gene expression levels. More convincingly, they selected a group of patients with very similar RAG1 expression levels and compared their ESC levels. Even with almost identical RAG gene expressions, relapsed patients still had significantly higher ESC copies than non-relapsed patients (P = 0.0156).
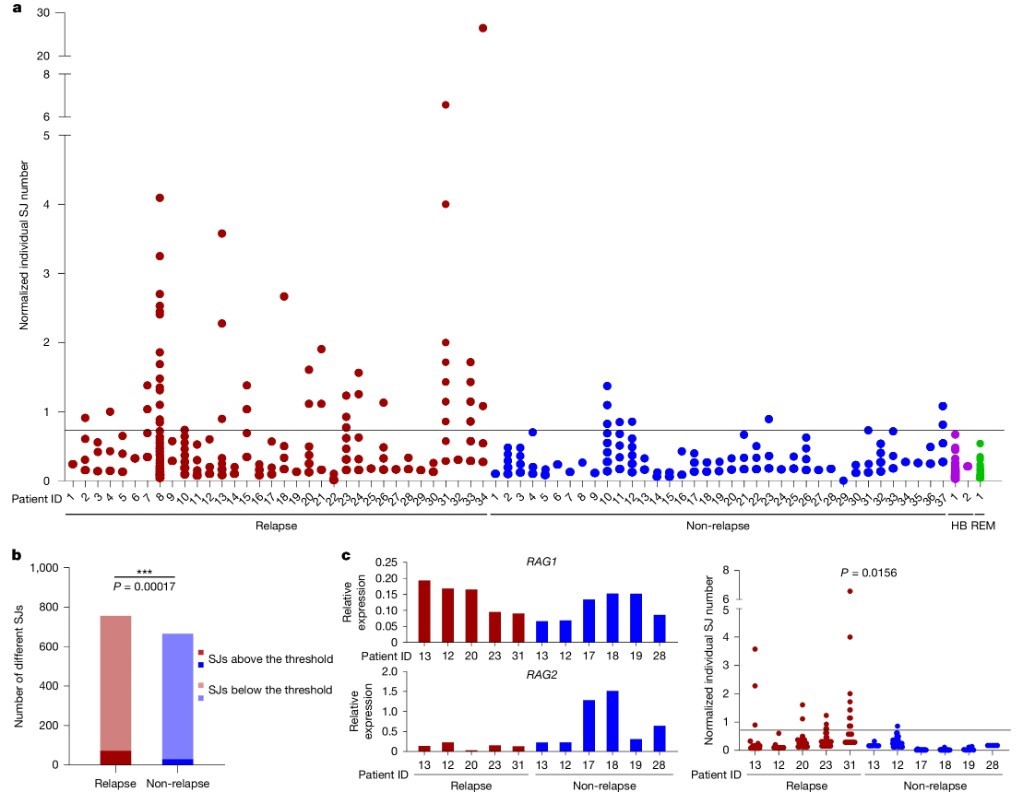
Fig2. High SJ copy numbers at diagnosis correlate with subsequent relapse.
This control experiment powerfully ruled out the "high RAG expression leads to more ESC" explanation. It strongly suggests that there’s an intrinsic biological characteristic within relapse-prone leukemia cells, either leading to abnormal ESC accumulation or with ESCs playing a more active role. Regardless, the core mystery seemingly points to ESCs themselves. These "cellular dust" are by no means innocent bystanders.
The Secret of the Ghost: Self-replicating Rogue Circular DNA
The surprising link between ESC quantity and relapse risk forces a reevaluation of a fundamental question: if ESCs cannot self-replicate, how do they maintain such high copy numbers in rapidly dividing cancer cells? The only reasonable explanation might be: these ESCs are indeed self-replicating!
This hypothesis, while bold, addresses the core issue. To verify it, the research team again demonstrated their clever experimental design. They utilized a characteristic of V(D)J recombination: whenever an ESC is excised and formed, it leaves a corresponding, unique "footprint"—a coding joint—on the main chromosome.
Consider this: if an ESC is "recently" formed, the cell should only have one coding joint "footprint". If formed "long ago", after multiple cell divisions, this "footprint" would also be replicated multiple times, resulting in many identical "footprints" in the cell. Now, if the ESC itself can replicate, even for those "recently" formed ESCs (with just one "footprint"), we should detect multiple ESC copies.
Based on this logic, researchers focused on the weakest signals in patient samples, likely representing "recently" formed ESCs. They compared these "freshly produced" ESC copies (via SJ quantification) and their corresponding chromosomal "footprints" (via coding joint quantification).
Results provided robust evidence for their hypothesis. Among relapse-prone patients, the ratio of these "new" ESC counts to their "footprint" counts was significantly higher than those in non-relapsed patients (P = 0.004). This proves that in relapse-prone leukemia cells, ESC replication is abnormally active. These circular DNAs aren't passively waiting for dilution; they're actively and efficiently "propagating".
What intrinsic factors drive the frantic replication of ESCs? To look for clues, researchers turned to gene expression analysis. Using RNA sequencing data from 123 BCP-ALL patients in a public database (74 relapsed), they performed a large-scale gene set enrichment analysis (GSEA). They found that in samples from inevitably relapsing patients, the gene set associated with "DNA REPAIR" was significantly upregulated (P = 0.0095).

Fig3. ESCs persist at higher copies in patients who subsequently relapse
They then validated the expression of several key genes related to DNA replication and repair, such as PCNA , POLE3 , POLE4 , and RBX1 , in their patient samples using RT-qPCR. The results confirmed that in patients with high ESC levels with relapse tendencies, expression levels of these genes were significantly higher than those with low ESC levels.
Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| PCNA-1579H | Recombinant Human PCNA, His-tagged | E.coli | Human | His | 8-256aa | |
| PCNA-4863H | Recombinant Human PCNA, His-tagged | E.coli | Human | His | Full Length | |
| PCNA-536H |
Recombinant Human Proliferating Cell Nuclear Antigen

|
E.coli | Human | Non |
|
|
| POLE3-28362TH | Recombinant Human POLE3, His-tagged | E.coli | Human | His | Full L. |
|
| POLE3-1220H | Recombinant Human POLE3 protein, GST-tagged | E.coli | Human | GST | 1-147 aa |
|
| POLE3-3326R | Recombinant Rhesus Macaque POLE3 Protein, His (Fc)-Avi-tagged | HEK293 | Rhesus macaque | Avi&Fc&His |
|
|
| POLE4-1831H | Recombinant Human POLE4, His-tagged | E.coli | Human | His | 1-117aa | |
| POLE4-13077M | Recombinant Mouse POLE4 Protein | Mammalian Cells | Mouse | His |
|
|
| RBX1-2227H | Recombinant Full Length Human RBX1, His-tagged | E.coli | Human | His | Full L. 1-108aa | |
| RBX1-1980HFL | Recombinant Full Length Human RBX1 Protein, C-Flag-tagged | Mammalian Cells | Human | Flag | Full L. |
|
Now, a clear molecular picture begins to emerge: in relapse-prone leukemia cells, there's an "intrinsic" abnormal state. This state is characterized by an overactive DNA replication and repair system, facilitating cancer cell growth and seemingly unintentionally opening a "convenience door" for ESC replication. These supposed to be cleared "trimmings" are allowed to replicate massively in this permissive environment, eventually forming a large "ghost army" lurking within the nucleus.
Now that this army has gathered, what will be their next move?
The Crime Scene: Unraveling the Layers of Genetic Mutations
We already know two major "dangerous factors" exist in high relapse-risk BCP-ALL cells: 1) Reactivated genetic scissors—RAG proteins; 2) Abundantly self-replicating circular DNA—ESCs. What deadly "spark" might occur when these two meet?
Previous studies have revealed that the RAG-ESC complex possesses a unique destructive ability known as the "cut-and-run" mechanism. During this process, RAG proteins bind with ESCs, forming an unstable complex. This complex acts as a rogue "genetic cruise missile", no longer neatly positioned at the immunoglobulin locus but wandering the genome. When it encounters sequences in the genome resembling V(D)J recombination signal sequences (RSSs)—called "cryptic RSS" (cRSS)—it performs an erroneous "cut" there.
This cut is characterized by causing a DNA double-strand break (DSB) on one side of the cRSS, leaving the other side of the genome intact. Such "one-sided" breaks are highly prone to errors during repair, leading to gene deletions, insertions, or translocations—structural variants (SVs). Hence, "one-sided cRSS breakpoint" becomes the unique "crime signature" left by the RAG-ESC complex.
If the RAG-ESC complex is indeed the culprit causing relapse, we should find more of these "crime characteristics" in the genomes of relapsed patients.
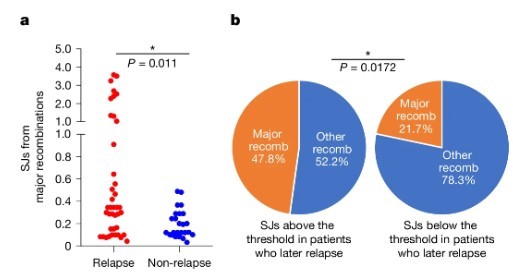
Fig4. SVs per patient at diagnosis with a single cRSS (left) or two cRSSs (right) at the breakpoints plotted for patients who later relapsed (121 patients) versus those who remained in remission
The research team used a large public database (TARGET) to analyze whole genome sequencing data from 150 BCP-ALL patients (121 relapsed, 29 non-relapsed) at diagnosis. They meticulously examined the breakpoint of each gene structural variant to check for cRSS presence.
The analysis results provided unequivocal evidence. Compared to the non-relapsed group, relapsed patients had a significantly higher number of structural variants containing "one-sided cRSS" in their genomes (P = 0.016). This directly links ESC-mediated "cut-and-run" gene damage with leukemia's relapse tendency.
The analysis didn't stop there. Researchers further probed: Are these gene mutations triggered by ESC randomly distributed, or do they have specific targets? They focused on a group of "relapse-related genes" frequently mutated during BCP-ALL relapse, such as IKZF1 , CREBBP , NT5C2 .
Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| IKZF1-125H | Recombinant Human IKZF1 protein, MYC/DDK-tagged | HEK293 | Human | DDK&Myc |
|
|
| IKZF1-182H | Recombinant Human IKZF1 protein, T7/His-tagged | E.coli | Human | His&T7 |
|
|
| CREBBP-1022R | Recombinant Monkey Crebbp Protein, His tagged | HEK293 | Monkey | His | ||
| CREBBP-001H | Recombinant Human CREBBP Protein, GST-tagged | E.coli | Human | GST | ||
| Crebbp-168M |
Active Recombinant Mouse Crebbp, His-tagged
|
Sf21 Cells | Mouse | His |
|
|
| CREBBP-192H |
Active Recombinant Human CREBBP, GST-tagged
|
Sf9 Cells | Human | GST | 1319-1710 a.a. |
|
| CREBBP-2815H |
Recombinant Human CREBBP protein, His&FLAG-tagged
|
E.coli | Human | Flag&His | 1081-1197 aa |
|
| NT5C2-2490H |
Recombinant Human 5'-Nucleotidase, Cytosolic II, His-tagged

|
E.coli | Human | His | 1-561aa | |
| NT5C2-1381H | Recombinant Human NT5C2, GST-tagged | E.coli | Human | GST | 209-561aa | |
| NT5C2-3473H | Recombinant Human NT5C2 protein, His-tagged | E.coli | Human | His | 209-561 aa |
When they concentrated analysis on these key genes, an even more shocking fact emerged. Data shows that "one-sided cRSS" mutation events within these "relapse-related genes" occur at a much higher frequency than the average level in the genome (P = 0.0001). This is not random damage but precise "targeted strikes". The RAG-ESC "cruise missile" seemingly selectively bombarded key strongholds that help cancer cells evade chemotherapy, promoting their survival and proliferation.
To establish a more direct causal link, researchers grouped patients based on RAG1 expression levels and ESC levels. They found that in the group with "high RAG1 expression" and "high ESC levels", the enrichment of "one-sided cRSS" mutations within relapse-related genes reached a peak (P = 9.44e-07). In groups with low RAG1 expression or low ESC levels, this phenomenon was less apparent.
The last hit almost locks in the "crime" of the RAG-ESC complex. It clearly outlines the complete logical chain from molecular events to clinical outcomes: High levels of ESCs and highly active RAG proteins join forces to produce numerous mutations through the "cut-and-run" mechanism, inflicting genetic damage closely tied to relapse, endowing leukemia cells with enhanced survival advantages, finally leading to chemotherapy failure and disease relapse.
At this point, the long-standing "cellular cold case" finally unveils the truth.
Old Enemies, New Insight: Reshaping the Perception Map of Leukemia Relapse
This landmark study ushers in a new paradigm for understanding leukemia relapse. It elevates a long-ignored cellular by-product—ESC—from a "harmless bystander" to a "central culprit".
Now we can construct a new model for BCP-ALL relapse:
In relapse-prone patients, a malignant cycle unfolds. Abnormally active RAG proteins drive not only leukemia onset but also continuously produce new ESCs. Concurrently, certain unknown factors inside these cancer cells (possibly linked to excessive activation of the DNA replication/repair system) foster a "hotbed" for ESC self-replication, causing an explosive accumulation of ESCs. These vast ESCs combine with RAG proteins to form a perennial "factory" producing gene mutations. This factory incessantly attacks genes crucial for cancer cell survival. Accumulating over time, it ultimately selects a batch of "super cancer cells" impervious to chemotherapy, with extraordinary vitality. These cells survive the initial treatment onslaught and finally grow strong, leading to disease relapse.
In contrast, in patients less prone to relapse, although ESCs are still produced, lacking effective replication mechanisms keeps their numbers low. Consequently, RAG-ESC complexes cause limited genetic damage, and cancer cell "evolution" is slower, making them more easily eradicated by standard chemotherapy, achieving long-term cure.
Establishing this new model signifies more than satisfying scientific curiosity. It brings tangible hope to clinical practice. Given the strong correlation between high ESC levels at diagnosis and future high relapse risk, measuring ESC copy numbers holds the potential to become a powerful new prognosis biomarker.
We can envision a future clinical scenario: along with routine examination, every newly diagnosed BCP-ALL child undergoes ESC level detection in bone marrow samples. Based on ESC "readouts", doctors accurately stratify patients into "high relapse risk" and "low relapse risk" groups. For low-risk patients, standard treatments with relatively mild side effects can continue; for high-risk patients with exceptionally high ESC levels, aggressive strategies can be decisively undertaken at the outset, such as escalating chemotherapy intensity, introducing targeted drugs, or even considering stem cell transplantation earlier, effectively stifling relapse risks in the cradle.
Of course, a long path lies ahead from breakthrough basic research to widespread clinical application. Larger-scale clinical trials are needed to validate the universality and accuracy of this biomarker, and faster, more economical ESC detection methods need to be developed. However, no matter how, the research has already opened a new window for us. It shows that behind the seemingly chaotic world of cancer cells, lies a precise order that can be understood and utilized.
The story of the "rogue DNA circle" also reminds us that in the complex system of life, there is no genuine "junk". Each overlooked corner may harbor clues crucial to destiny. Scientific exploration is that never-ending great adventure chasing between the "unknown" and the "known". And this time, we seem to have moved closer to the light illuminating the futures of children with leukemia.
Related Products & Services
- Cytokines for Organoid Culture
- ADC Target Protein
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Immune Checkpoint Proteins
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Gao, Z., Scott, J. N., Edwards, M. P., Casey, D., Wang, X., Gillen, A. D., Ryan, S., Russell, L. J., Moorman, A. V., De Tute, R., Cargo, C., Ford, A. M., Westhead, D. R., & Boyes, J. (2025). Excised DNA circles from V(D)J recombination promote relapsed leukaemia. Nature, 1-10. https://doi.org/10.1038/s41586-025-09372-6
