Fluorescent-labeled Recombinant Proteins

🧪 TNFRSF17-2348HP
Source: HEK293
Species: Human
Tag: Fc&His
Conjugation: R-PE
Protein Length:

🧪 CD19-3307HP
Source: HEK293
Species: Human
Tag: Fc
Conjugation: R-PE
Protein Length:

🧪 Erbb2-4097RP
Source: HEK293
Species: Rat
Tag: Fc
Conjugation: R-PE
Protein Length:

🧪 IL13RA2-618HP
Source: Mammalian Cells
Species: Human
Tag: Fc
Conjugation: R-PE
Protein Length:

🧪 IL13RA2-765HP
Source: HEK293
Species: Human
Tag: Fc&His
Conjugation: R-PE
Protein Length:

🧪 SLAMF7-186HP
Source: HEK293
Species: Human
Tag: Fc
Conjugation: R-PE
Protein Length:

🧪 TNFRSF17-1817HP
Source: HEK293
Species: Human
Tag: Fc
Conjugation: R-PE
Protein Length:

🧪 TNFRSF17-2348HA
Source: HEK293
Species: Human
Tag: Fc&His
Conjugation: APC
Protein Length:
🧪 TNFRSF17-223HP
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Met1-Ala54
🧪 BTLA-1148RAF488
Source: HEK293
Species: Rat
Tag: Fc
Conjugation: Alexa Fluor 488
Protein Length: 384
🧪 BTLA-195CAF488
Source: HEK293
Species: Monkey
Tag: Fc
Conjugation: Alexa Fluor 488
Protein Length: Met1-Leu155, 366
🧪 CA9-1039CAF488
Source: HEK293
Species: Dog
Tag: His
Conjugation: Alexa Fluor 488
Protein Length: 384
🧪 CA9-1544CAF488
Source: HEK293
Species: Dog
Tag: Fc
Conjugation: Alexa Fluor 488
Protein Length: 614
🧪 CD160-453HAF488
Source: HEK293
Species: Human
Tag: His
Conjugation: Alexa Fluor 488
Protein Length: 143
🧪 CD19-3307HAF488
Source: HEK293
Species: Human
Tag: Fc
Conjugation: Alexa Fluor 488
Protein Length: Pro20-Pro278
SubCategories
Background
Overview
Fluorescent tag, also known as fluorescent label or fluorescent probe, is a molecule attached chemically to aid in detecting biomolecules such as a protein, antibody, or amino acid. Conjugating fluorescent labels to proteins or other biomolecules is a common means of studying the relative structure and function. Fluorescent proteins are advantageous due to the lack of free dye in solution, thus reducing the overall intensity changes observed with organic fluorophores. However, its large label size and limited labeling sites prevented efficient targeting.
The study of fluorescent protein provides an indispensable research means for life science. At present, there is still a need for further development in the depth of imaging in vivo, and new monomer near-red/red fluorescent protein molecules with high brightness need to be continuously improved and optimized. The development of fluorescent proteins with new characteristics, such as photoconversion and photoactivated fluorescent proteins and large strokes shift fluorescent proteins, will also bring new breakthroughs to optical imaging technology, effectively improving the spatio-temporal resolution and sensitivity of imaging.
Development history
As early as 1962, Japanese scientist Osamu Shimomura isolated green fluorescent protein from Aequorea victoria. This was the first fluorescent protein to be discovered, and it appears green under natural light and fluoresces strongly green under ultraviolet light. Because GFP can fluoresce spontaneously in organisms, it has become an important tool in molecular and cell biology research. Scientists can use GFP to label specific proteins and thus observe the distribution and dynamics of these proteins within the cell.
The 2008 Nobel Prize in Chemistry was awarded to the American scientists Osamu Shimomura and Martin. Martin Chalfie and Roger Y. Tsien for their outstanding contributions to biofluorescence. Shimomura isolated and purified medusin and green fluorescent protein. Martin Chaffey demonstrates the scientific value of green fluorescent protein as a bright genetic tag for a variety of biological phenomena. Qian greatly facilitates the application of green fluorescent protein technology, explains its mechanism, and discovers many new colors from red to blue and corresponding application tools.
Common fluorescent proteins
With the discovery of GFP, scientists genetically engineered hundreds of GFP mutants that fluoresce in different colors, from blue to red. In addition, fluorescent proteins from other Marine organisms, such as sea anemones and corals, extend the emission spectrum to the far infrared region. This variety of colors gives scientists more options, allowing them to pick the most appropriate fluorescent label for their experimental needs.
mGFP5: By replacing ser65 of wild-type GFP with thr, the fluorescence intensity is 4-6 times higher than that of wild-type GFP. The excitation wavelength of GFP is close to the ultraviolet region, which has higher optical requirements and greater toxicity to organisms, so it is not suitable for observation of living cells.
EGFP: Phe64 is replaced with Leu on the basis of mGFP5, and the resulting GFP mutant is named EGFP. The fluorescence of EGFP chromophore is greatly improved at 37℃, and the fluorescence intensity is 35 times higher than that of wild-type GFP, and can still be stably detected 16-24 hours after excitation. EGFP is still the most commonly used green fluorescent protein.
mRFP1: The first-generation monomer red fluorescent protein mRFP1 is derived by modifying 33 residues of DsRed, with an excitation wavelength of 584 nm and an emission wavelength of 607 nm. Like other fluorescent protein derivatives, mRFP1 also exhibited significant fluorescence emission reduction and photobleaching.
mCherry: A new fluorescent protein was obtained by point mutation of chromophore residues of mRFP1, named mCherry after the corresponding fruit name, and the emission peak was located at 610 nm. mCherry ripens quickly, has good monomer properties, strong light stability, but low brightness.
Applications
Fluorescent proteins have a wide range of applications, including immunohistochemistry, fluorescence in situ hybridization (FISH), cell tracking, receptor labeling, and fluorescence spectroscopy.
- Cell imaging: Fluorescently labeled proteins can be used to observe structural and dynamic changes within cells, such as the nucleus, organelles, signaling pathways, etc.
- Protein interaction studies: By fusing fluorescently labeled proteins with target proteins, interactions between proteins and complex formation can be observed.
- Quantitative PCR: Fluorescently labeled proteins can be used in real-time quantitative PCR experiments to quantify the expression level of target genes by detecting changes in the fluorescence signal.
- Live cell sorting: Fluorescent-labeled proteins can be used for live cell sorting to separate cells or subcell populations by specifically binding to target molecules.
- Immunofluorescence staining: Fluorescently labeled proteins can be used in immunofluorescence staining experiments to detect specific protein expression in tissues or cells by specifically binding antibodies.
Case Study
Case Study 1: CD19-3307HP
Autologous chimeric antigen receptor (CAR) T cells targeting the CD19 antigen have demonstrated a high complete response rate in relapsed/refractory B-cell malignancies. However, autologous CAR T cell therapy is not an option for all patients. Here we optimized conditions for clinical-grade manufacturing of allogeneic CD19-CAR T cells using CD45RA-depleted donor memory T cells (Tm) for a planned clinical trial. Tm were activated using the MACS GMP T Cell TransAct reagent and transduced in the presence of LentiBOOST with a clinical-grade lentiviral vector that encodes a 2nd generation CD19-CAR with a 41BB.zeta endodomain. Transduced T cells were transferred to a G-Rex cell culture device for expansion and harvested on day 7 or 8 for cryopreservation. The resulting CD19-CAR(Mem) T cells expanded on average 34.2-fold, and mean CAR expression was 45.5%. CD19-CAR(Mem) T cells recognized and killed CD19-positive target cells in vitro and had potent antitumor activity in an ALL xenograft model.
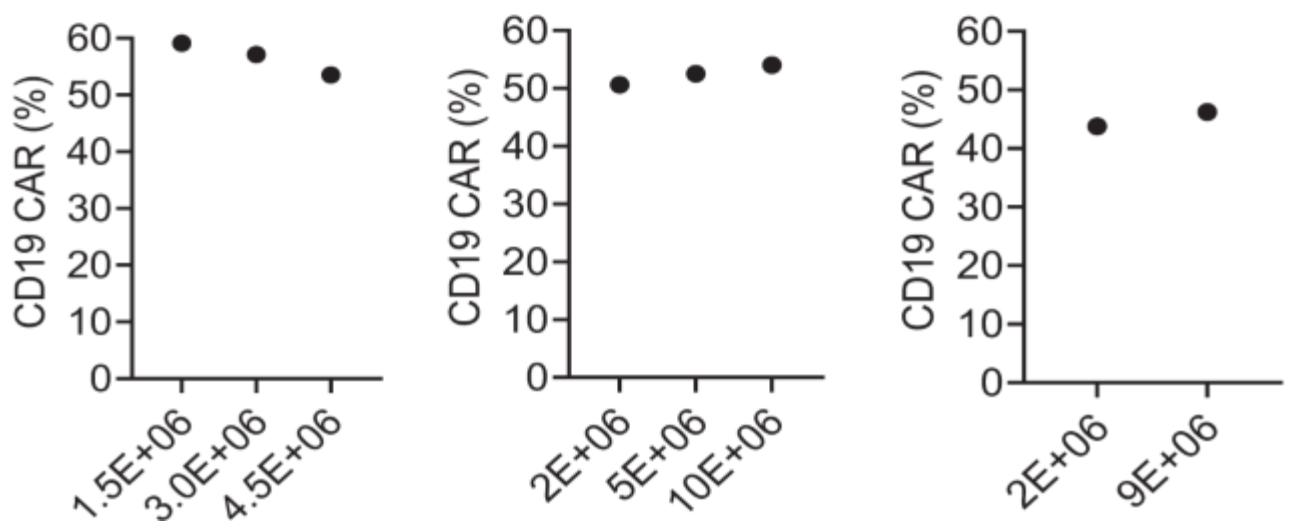
(Young-In Kim-Hoehamer, 2022)
Fig1. Percentage of CD19-CAR expression was determined by flow cytometry. Donor identifications are shown at the top of each column. Each column represents one donor.
Case Study 2: IL13RA2-765HP
T cells engineered with chimeric antigen receptors (CARs) show great promise in the treatment of some cancers. Modifying T cells to express CARs generally relies on T-cell transduction using viral vectors carrying a transgene, resulting in semi-random DNA integration within the T-cell genome. While this approach has proven successful and is used in generating the Food and Drug Administration (FDA, USA) approved B-lymphocyte antigen CD19-specific CAR T cells, it is possible the transgene could integrate into a locus that would lead to malignant transformation of the engineered T cells. In addition, manufacturing viral vectors is time-consuming and expensive. One way to overcome these challenges is site-specific gene integration, which can be achieved through clustered regularly interspaced short palindromic repeat (CRISPR) mediated editing and non-viral DNA, which serves as a template for homology-directed repair (HDR). This non-viral gene editing approach provides a rapid, highly specific, and inexpensive way to engineer T cells. The researchers then applied these established guidelines to target the TRAC or interleukin-13 (IL-13) locus for the knock-in of synthetic molecules, such as a CAR, bispecific T-cell engager (BiTE), and other transgenes. While integration efficiency depends on the targeted gene locus and selected transgene, this optimized protocol reliably generates the desired insertion at rates upwards of 20%.
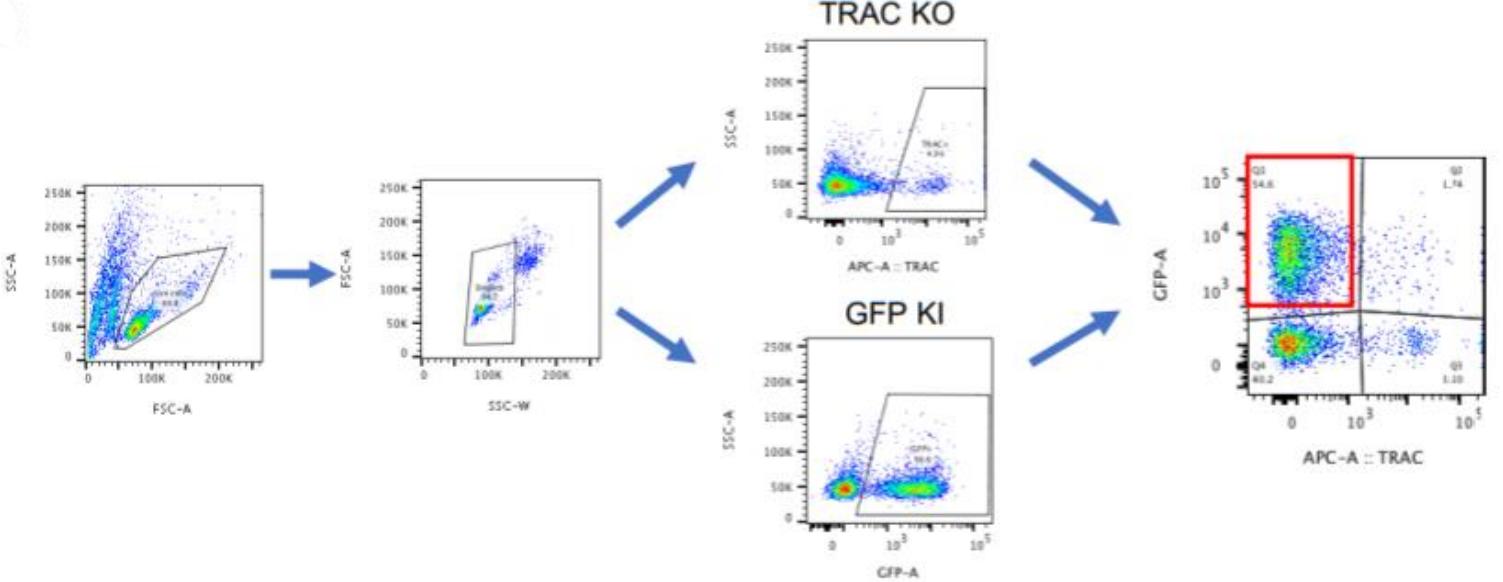
(Zelda Odé, 2020)
Fig2. IL-15.E2A.mClover3 transgene integration into TRAC locus. Gating strategy for detection of transgene expression in T cells by flow cytometry.
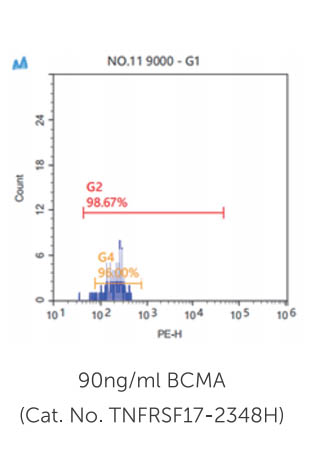
Case Study 3: TNFRSF17-2348HP
Chimeric antigen receptor (CAR) T-cell therapy remains limited to select centers that can carefully monitor adverse events. To broaden use of CAR T cells in community clinics and in a frontline setting, we developed a novel CD8+ CAR T-cell product, Descartes-08, with predictable pharmacokinetics for treatment of multiple myeloma. Descartes-08 is engineered by mRNA transfection to express anti-BCMA CAR for a defined length of time. Descartes-08 expresses anti-BCMA CAR for 1 week, limiting risk of uncontrolled proliferation; produce inflammatory cytokines in response to myeloma target cells; and are highly cytolytic against myeloma cells regardless of the presence of myeloma-protecting bone marrow stromal cells, exogenous a proliferation-inducing ligand, or drug resistance including IMiDs. The magnitude of cytolysis correlates with anti-BCMA CAR expression duration, indicating a temporal limit in activity. In the mouse model of aggressive disseminated human myeloma, Descartes-08 induces BCMA CAR-specific myeloma growth inhibition and significantly prolongs host survival (p < 0.0001).
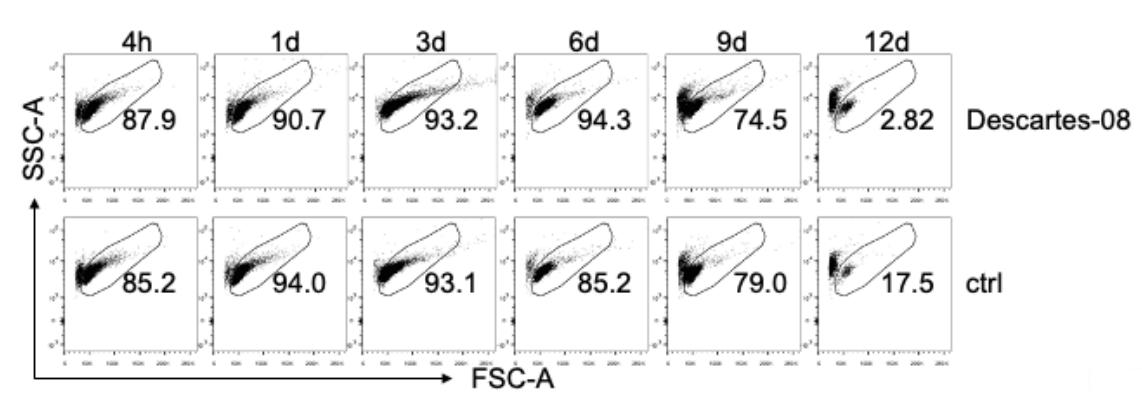
(Liang Lin, 2021)
Fig3. Using FCM analysis, cell viability after being thawed was evaluated by FSC-A and SSC-A at indicated time periods (4 hours to 12 days, 4h to 12d). CAR expression was determined by incubating CAR T cells with 0.4 µg/ml of PE-conjugated recombinant BCMA (Recombinant human BCMA/TNFRSF17 protein, Fc/His-tagged, R-PE labeled; Creative Biomart, Shirley, NY) for 20 min at room temperature.
Case Study 4: Cd47-647MF
Nanocarriers (NCs) help improve the performance of therapeutics, but their removal by phagocytes in the liver, spleen, tissues, etc. diminishes this potential. Although NC functionalization with polyethylene glycol (PEG) lowers interaction with phagocytes, it also reduces interactions with tissue cells. Coating NCs with CD47, a protein expressed by body cells to avoid phagocytic removal, offers an alternative. Previous studies showed that coating CD47 on non-targeted NCs reduces phagocytosis, but whether this alters binding and endocytosis of actively-targeted NCs remains unknown. To evaluate this, we used polymer NCs targeted to ICAM-1, a receptor overexpressed in many diseases. Co-coating of CD47 on anti-ICAM NCs reduced macrophage phagocytosis by ∼50% for up to 24 h, while increasing endothelial-cell targeting by ∼87% over control anti-ICAM/IgG NCs. Comparable outcomes were observed for NCs targeted to PECAM-1 or transferrin receptor, suggesting broad applicability. When injected in mice, anti-ICAM/CD47 NCs reduced liver and spleen uptake by ∼30-50% and increased lung targeting by ∼2-fold (∼10-fold over IgG NCs).
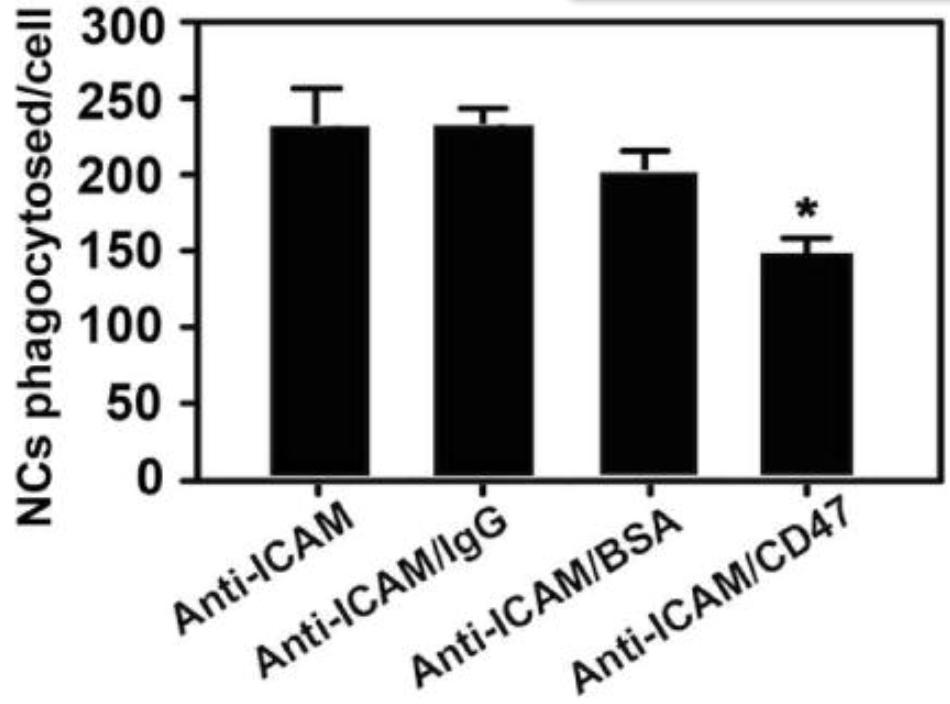
(Joshua Kim, 2017)
Fig4. Quantification of the uptake of 200 nm diameter model NCs consisting of green fluorescent polystyrene nanoparticles coated with anti-ICAM alone or 1:1 mass ratio mixtures of anti-ICAM and IgG, anti-ICAM and bovine serum albumin (BSA), or anti-ICAM and recombinant CD47.
Advantages
- Wide Coverage: More than 2000 fluorescent-labeled recombinant proteins from over 20 different species and nearly 15 different sources.
- High Quality: Identical binding capacity before and after conjugations, bioactivity verified by flow cytometry and protocol offered,and high batch-to-batch consistency.
- Experienced: We have professional research team with experience of many years in the field of molecular and cell biology.
- Fast turnaround: Under the premise of your protein expression and purification, as little as 4-6 weeks.
- Customized services: We develop research programs and produce desired products according to your specific needs.
FAQ
-
Q: How should I store the recombinant proteins??
A: Sample is stable for at least 12 months when stored at -20 to -80°C from date of manufacture. For longer storage, add equal volume of glycerol to the sample solution and store at -20 to -80°C. Avoid freeze-thaw cycles.
-
Q: How is the purity for the recombinant proteins determined?
A: Protein purity is most often determined by SDS-PAGE; sometimes, it is analyzed by HPLC as well. Please refer to either the product manual, data sheet or certificate of analysis (CoA) for product-specific purity levels and methods.
Resources
References
- Kim-Hoehamer, YI.; et al. Development of a cGMP-compliant process to manufacture donor-derived, CD45RA-depleted memory CD19-CAR T cells. Gene Ther. 2023;30(3-4):222-231.
- Odé, Z.; et al. CRISPR-Mediated Non-Viral Site-Specific Gene Integration and Expression in T Cells: Protocol and Application for T-Cell Therapy. Cancers (Basel). 2020;12(6):1704.
- Lin, L.; et al. Preclinical evaluation of CD8+ anti-BCMA mRNA CAR T cells for treatment of multiple myeloma. Leukemia. 2021;35(3):752-763.
- Kim, J.; et al. Co-coating of receptor-targeted drug nanocarriers with anti-phagocytic moieties enhances specific tissue uptake versus non-specific phagocytic clearance. Biomaterials. 2017;147:14-25.


