Uncategorized Tuesday, 2025/12/23
Introduction: A New Analysis of Drug-Genome Interactions
Research has identified several approved drugs as promising candidates for treating DNA repair-deficient cancers, offering potential options beyond current therapies.
In a new study, scientists at the Max Planck Institute for Evolutionary Anthropology analyzed the effects of more than 2,000 clinically approved drugs on DNA repair and CRISPR genome editing outcomes. They discovered compounds that could improve genome editing, molecules that selectively kill cultured cancer cells, and further identified new functions for two proteins in DNA repair.
How DNA Repair Impacts Genome Editing
DNA double-strand breaks are critical damage in the genome that can be repaired in multiple ways. Some repair processes are rapid but introduce additional mutations at the damage site, while others are slower but enable precise correction. In genome editing, these pathways can be harnessed to introduce mutations into human cells. This involves using programmable CRISPR-Cas gene scissors to cut DNA at specific genomic locations. The resulting breaks must be repaired by the cell for survival, and researchers can provide a DNA template carrying the desired mutation. The efficiency of integrating this mutation largely depends on the activity of repair pathways, making it necessary to develop tools to suppress competing pathways and improve the efficiency of achieving intended outcomes.
The Study: Mapping Drug Effects on DNA Repair
A team of scientists at the Max Planck Institute for Evolutionary Anthropology studied how FDA-approved drugs influence the choice of DNA repair pathways. "Understanding how everyday medications interact with CRISPR-based therapies will become increasingly important as these treatments move into real-world clinical use," said co-first author Dominik Macak of the study published in Nature Communications.
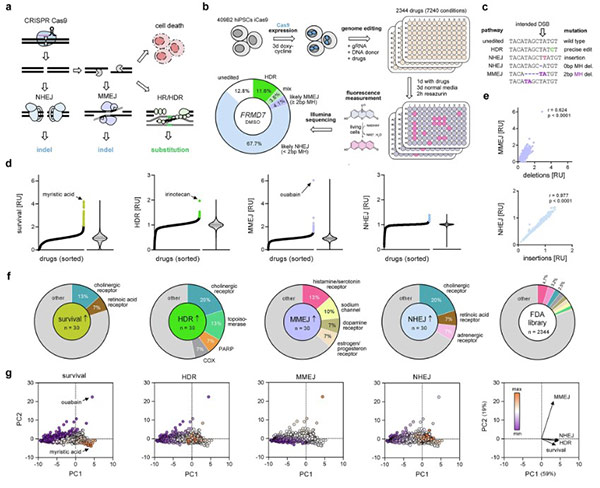
Fig1. Drug screening for DSB repair pathway modulators.
Drug Interactions and Clinical Implications
With the first CRISPR gene therapy approved in the United States, United Kingdom, and European Union in late 2023, patients receiving such treatments may also be taking common medications for infections or chronic diseases. Some of these routine drugs can affect cellular processes such as DNA repair, which may in turn affect therapy efficacy or safety.
Scientists created a comprehensive map showing how clinically approved drugs affect the way human cells repair broken DNA. They tested more than 7,000 drug conditions to determine how each compound alters DNA repair choice after targeted CRISPR cleavage. "We anticipate this catalog will be a valuable resource for clinicians and researchers working in disease modeling, gene therapy, and oncology," added co-first author Philipp Kanis.
The team identified several drugs capable of affecting major repair pathways. Using the screening data, they further explored previously unrecognized drug targets that have the greatest impact on repair outcomes. Notably, they discovered new roles in DNA repair for two proteins not previously linked to genome editing. These proteins are estrogen receptor 2 (ESR2) and aldehyde oxidase 1 (AOX1). Targeted inhibition of ESR2 can increase precise editing efficiency by up to fourfold, while drugs inhibiting AOX1 can be used to kill cultured cancer cells lacking one repair pathway—a condition applicable to many cancer cells.
Our Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| ESR2-12558H | Recombinant Human ESR2, GST-tagged | E.coli | Human | GST | N-term-323a.a. | |
| ESR2-103H | Recombinant Human Estrogen beta receptor LBD/Hsp90 complex, GST-tagged | Sf9 Cells | Human | GST |
|
|
| ESR2-122H | Recombinant Human ESR2, GST-tagged | Sf9 Cells | Human | GST |
|
|
| ESR2-1506R | Recombinant Rhesus monkey ESR2 Protein, His-tagged | Mammalian Cells | Rhesus macaque | His |
|
|
| AOX1-18H | Recombinant Human AOX1 protein, MYC/DDK-tagged | HEK293 | Human | DDK&Myc |
|
|
| AOX1-300C | Recombinant Cynomolgus AOX1 Protein, His-tagged | Mammalian Cells | Cynomolgus | His |
|
|
| Aox1-3266M | Recombinant Mouse Aox1, His-tagged | E.Coli/Yeast | Mouse | His | 1333 |
|
| AOX1-3316C | Recombinant Chicken AOX1 | Mammalian Cells | Chicken | His |
|
|
| AOX1-341R | Recombinant Rhesus monkey AOX1 Protein, His-tagged | Mammalian Cells | Rhesus macaque | His |
|
|
| AOX1-694R | Recombinant Rat AOX1 Protein | Mammalian Cells | Rat | His |
|
Therapeutic Potential for Cancer Treatment
"Our study identifies several approved drugs as promising candidates for treating DNA repair-deficient cancers, offering potential options beyond current therapies," said senior researcher Stephan Riesenberg. "However, further research is needed to validate whether our findings from cultured cell experiments can truly be translated into real-world medical applications."
Future Directions and Validation
The researchers emphasize that while their in vitro findings are promising, clinical trials will be necessary to confirm the safety and efficacy of repurposing these existing medications for CRISPR-enhanced therapies and cancer treatment.
Related Products & Services
- Cell and Gene Therapy
- Immune Checkpoint Proteins
- ADC Target Protein
- PROTAC Targets
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Dominik Macak et al, Repurposing clinically safe drugs for DNA repair pathway choice in CRISPR genome editing and synthetic lethality, Nature Communications (2025). DOI: 10.1038/s41467-025-67243-0.
