Uncategorized Thursday, 2026/01/22
Pharmacological inhibition of USP25 using the small-molecule inhibitor AZ1 significantly alleviates PD symptoms in mice, suggesting that USP25 suppression may represent a potential therapeutic strategy for PD.
Parkinson's disease (PD) is a progressive neurodegenerative disorder that poses a major threat to global health. Identifying therapeutic targets for PD will facilitate more effective clinical treatments.
The Wang Xu research group from Wenzhou Medical University published online in PNAS a study titled "USP25 inhibition ameliorates Parkinson’s disease by restoring mitophagy." This study reveals that ubiquitin-specific protease 25 (USP25) exacerbates dopaminergic neuron loss and motor dysfunction in mouse models of PD by disrupting the mitophagy machinery.
The study found that PD-associated Usp25 variants enhance USP25 expression. Genetic knockout or knockdown of USP25 significantly ameliorates PD-like pathological changes and motor dysfunction in mouse PD models induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or the human α-synuclein mutant A53T.
Mechanistic studies demonstrated that USP25 disrupts the mitophagy machinery in neurons by interacting with the key mitophagy adaptor protein optineurin (OPTN) and impairing its binding to K63-linked polyubiquitin chains. Pharmacological inhibition of USP25 using the small-molecule inhibitor AZ1 significantly alleviates PD symptoms in mice, suggesting that USP25 suppression may represent a potential therapeutic strategy for PD.
PD is the second most common neurodegenerative disease worldwide, affecting over six million people. Its pathological hallmarks include the accumulation of phosphorylated and misfolded α-synuclein in Lewy bodies (LBs) and Lewy neurites (LNs), as well as progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc). Clinically, PD causes a range of symptoms including bradykinesia, rigidity, tremor, postural instability, cognitive decline, and autonomic dysfunction. Current PD treatments primarily focus on alleviating motor and non-motor symptoms, while disease-modifying pharmacological therapies remain unavailable.
Our Related Proteins
Although most PD cases are sporadic and aging is the greatest risk factor, both genetic and environmental factors increase disease risk and promote progression. For example, mutations in genes encoding α-synuclein , parkin , DJ-1 , leucine-rich repeat kinase 2 (LRRK2) , PTEN-induced putative kinase 1 (PINK1) , and Rab-32 are associated with PD.
Notably, parkin, encoded by the PRKN gene, is an enzyme that specifically regulates ubiquitination by covalently attaching ubiquitin proteins to lysine residues of substrate proteins. The ubiquitination process is sequentially catalyzed by E1 ubiquitin-activating enzymes, E2 ubiquitin-conjugating enzymes, and E3 ubiquitin ligases, and is reversely regulated by deubiquitinating enzymes (DUBs). When mitochondrial damage is irreparable, PINK1 recruits and activates the E3 ligase parkin, which assembles K6-, K11-, K48-, and K63-linked polyubiquitin chains on outer mitochondrial membrane (OMM) proteins.
These polyubiquitin chains are then recognized by ubiquitin-binding mitophagy receptors, leading to engulfment of damaged mitochondria by autophagosomes. Numerous previous studies have demonstrated that DUBs significantly influence PD pathogenesis and progression by regulating ubiquitination-dependent degradation of misfolded proteins and damaged organelles. Recently, the gene Usp25 encoding USP25 was identified as a PD risk gene locus. However, the function of USP25 in PD remains unclear.
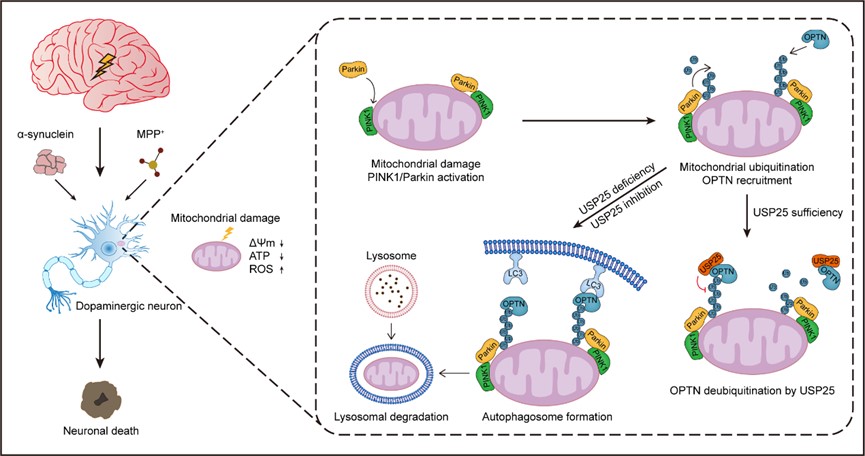
Fig1. USP25 impairs mitophagy by deubiquitinating OPTN, thereby exacerbating Parkinson's disease
This study found that PD-associated single nucleotide polymorphisms (SNPs) at the Usp25 gene locus enhance USP25 expression. Genetic knockout or pharmacological inhibition of USP25 significantly reduces mouse susceptibility to MPTP-induced dopaminergic neuron loss and motor dysfunction. Functionally, USP25 promotes stress-induced neuronal death by impairing mitophagy.
USP25 disrupts the mitophagy machinery by interacting with OPTN and reducing its binding to K63-linked polyubiquitin chains. Furthermore, USP25 knockdown alleviates PD-like symptoms and pathological changes in transgenic mice carrying the human α-synuclein mutant A53T. These findings elucidate the function and molecular mechanism of USP25 in PD, highlighting the potential of USP25 as a novel therapeutic target for PD.
Related Products & Services
- Neurodegenerative Disease
- Adhesion Molecules
- Blood-brain Barrier Permeability
- Neurotrophic Factors and Receptors
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Xu, Y., Jin, K., Chen, J., Li, Z., Zhu, Z., Su, X., Shen, J., Zhou, B., Cao, Z., Lou, L., Deng, D., Zhang, J., Liu, B., Shentu, Y., & Wang, X. (2026). USP25 inhibition ameliorates Parkinson’s disease by restoring mitophagy. Proceedings of the National Academy of Sciences, 123(2), e2516471123. https://doi.org/10.1073/pnas.2516471123

