Uncategorized Tuesday, 2025/12/23
Keywords: Vitamin B6, Cancer immunotherapy, CD8+ T cells
Key Discovery: Vitamin B6 Maintains T-Cell Stemness
Vitamin B6, through its active form pyridoxal 5'-phosphate (PLP), directly inhibits the p70S6K kinase, preventing its phosphorylation of the BACH2 transcription factor, thereby maintaining a stem-like state in CD8⁺ T cells, delaying their exhaustion, and significantly enhancing their anti-tumor capacity.
In the tumor battlefield, CD8+ T cells are like special forces operating deep behind enemy lines, tasked with identifying and destroying tumor cells. However, under prolonged, high-pressure conditions, many CD8+ T cells fall into an "exhausted" state, lose their combat effectiveness, stop proliferating, and are eventually overwhelmed by the tumor.
Yet, a small group of "elite" cells can maintain a "stem-like" state, capable of both self-renewal and long-term persistence while continuously differentiating into new "combat" units. What determines their fate?
Recently, a research team from Tsinghua University published a study in the Cell journal Developmental Cell that reveals a new breakthrough for vitamin B6 in the longstanding challenge of cancer immunotherapy.
The study shows that vitamin B6, through its active form PLP, directly inhibits the p70S6K kinase, preventing its phosphorylation of the BACH2 transcription factor. This maintains a stem-like state in CD8⁺ T cells, delays their exhaustion, significantly enhances their anti-tumor capacity, and also boosts the efficacy of immune checkpoint inhibitors (such as anti-PD-1 therapy).
Our Related Proteins
Tracing the Source: How PLP Works
The research team first observed in a melanoma mouse model that vitamin B6—an essential nutrient found in leafy greens, nuts, and fish—can significantly slow tumor growth.
The real "hero" responsible for this effect is pyridoxal 5'-phosphate (PLP), the active form of vitamin B6 in the body. It not only has a preventive effect before tumor formation, but even when supplementation begins after tumors have already grown, it can still effectively inhibit tumor growth and extend mouse survival.
Moreover, PLP significantly improved both the quantity and functional status of CD8⁺ T cells in the tumor microenvironment, promoting their infiltration into tumor tissue while enhancing their proliferative capacity and cytokine secretion levels.
To confirm PLP's central role, the team conducted in-depth research and discovered that PDXK—the key enzyme responsible for synthesizing PLP—is significantly downregulated in various human tumor tissues.
In colon cancer patients, low PDXK expression is closely associated with poorer prognosis. At the animal level, researchers generated mice with heterozygous PDXK deficiency, and these mice developed tumors that grew faster and larger.
Supplementation with exogenous PLP almost completely reversed this disadvantage.
So how does PLP work? Through antibody-mediated specific depletion experiments, the research team discovered a key fact: PLP affects CD8+ T cells in the tumor microenvironment. Once CD8+ T cells were depleted, PLP's anti-tumor effect completely disappeared; depleting other immune cells had no such impact. CD8+ T cells "trained" with PLP in vitro and then adoptively transferred into tumor-bearing mice demonstrated greater persistence and superior tumor-suppressive effects.

Fig1. Tumor-Infiltrating CD8⁺ T Cells Are Key to PLP's Tumor-Suppressive Effect
What "magic" does PLP inject into T cells? The researchers delved deep into the cells and found that CD8+ T cells treated with PLP show significantly elevated "stem-like" characteristic markers while corresponding decreases in "exhaustion" markers.
These cells appear to be "frozen" in a more potent "youthful state." This change was validated not only in multiple mouse tumor models but also observed consistently in human CD8⁺ T cells from healthy individuals, with no gender differences.

Fig2. PLP Maintains a Stem-Like Phenotype in CD8⁺ T Cells and Enhances Their Anti-Tumor Function
Identifying the Molecular Target
Tracing to the source, the research team began searching for PLP's direct molecular target within cells. After extensive screening, they identified p70 ribosomal protein S6 kinase (p70S6K). Like a specially crafted key, PLP can insert into p70S6K's ATP-binding pocket, bind to this kinase that provides an "acceleration signal" for cell growth, and thereby inhibit its activity.
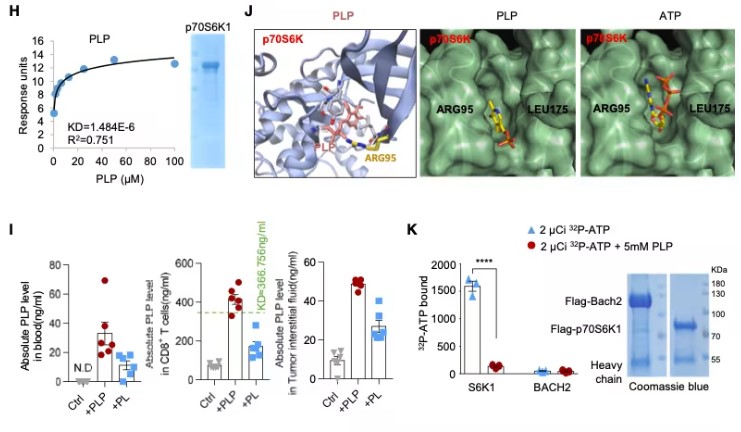
Fig3. p70S6K Is the Natural Target of PLP
One of p70S6K's downstream targets is the transcription factor BACH2, which is crucial for maintaining T-cell stemness. The researchers found that p70S6K phosphorylates serine 520 of the BACH2 protein. This phosphorylation acts like an "exile order," causing BACH2 to be expelled from the nucleus and preventing it from entering the "command center" to initiate the gene program that maintains stemness. By inhibiting p70S6K, PLP protects BACH2, allowing it to remain in the nucleus and perform its function.
Powerful Combination: Synergy with PD-1 Inhibitors
The findings of this study also hold significant clinical translational value. When researchers combined PLP with the currently popular immune checkpoint inhibitor anti-PD-1 antibody, they observed remarkable synergistic effects in mouse models.
Compared to either treatment alone, the combination therapy more effectively controlled tumor growth and yielded higher complete remission rates and survival. This provides a highly promising, safe, and economical combination strategy for overcoming resistance to PD-1 therapy and improving response rates in the clinic.

Fig4. PLP Regulates CD8⁺ T Cell Phenotype by Inhibiting p70S6K-Mediated BACH2 Phosphorylation and Synergizes with Anti-PD-1 Therapy to Suppress Tumors
Future Directions and Remaining Questions
Of course, this study also leaves puzzles to be explored. For example, the classic downstream function of p70S6K is regulating protein translation, yet PLP's ability to maintain T-cell stemness does not appear to depend on this function. Could there be more refined, selective regulation at play?
Although the research team has validated PLP's effects in human T cells, its efficacy and optimal application in real cancer patients still await final answers from future clinical trials.
Related Products & Services
- Immune Checkpoint Proteins
- ADC Target Protein
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Wu, J., Li, G., Zhou, J., Sun, X., Wang, H., Gong, H., & Jiang, P. (2026). Vitamin B6 preserves the stemness-like phenotypes and antitumor ability of CD8⁺ T cells. Developmental Cell, 61(3), 1-16. https://doi.org/10.1016/j.devcel.2025.10.017
