Uncategorized Tuesday, 2025/12/23
Keywords: Immunotherapy, Melanoma cells, T-cell receptor (TCR), TCR sequencing (TCR-seq)
Although melanoma cells often express numerous mutated proteins, infiltration of reactive T cells rarely leads to tumor eradication by the immune system.
A research team led by Carmit Levy at Tel Aviv University, Israel, published an online study in Cell titled "HLA export by melanoma cells decoys cytotoxic T cells to promote immune evasion." The study discovered that large extracellular vesicles secreted by melanoma cells—called melanosomes—are decorated with major histocompatibility complex (MHC) molecules that stimulate CD8+ T cells through T-cell receptors (TCRs), leading to T-cell dysfunction and apoptosis.
Immunopeptidomic and TCR sequencing (TCR-seq) analyses revealed that these melanosomes carry MHC-bound tumor-associated antigens with higher affinity and immunogenicity, competing with tumor cells by directly engaging in TCR-MHC interactions. Biopsy analyses of melanoma patients confirmed that melanosomes can trap infiltrating lymphocytes, induce partial activation, and reduce CD8+ T-cell cytotoxicity. Inhibition of melanosome secretion in vivo significantly reduces tumor immune evasion. These findings demonstrate that MHC export protects melanoma from T-cell cytotoxicity.
In summary, the study highlights a novel immune evasion mechanism and proposes a therapeutic avenue to enhance anti-tumor immunity.
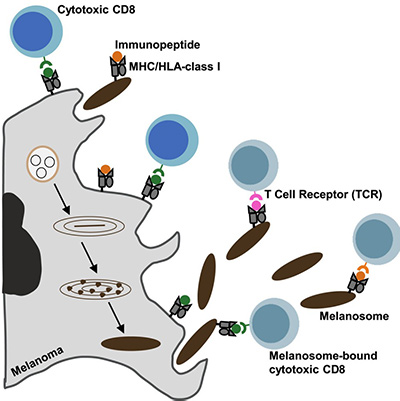
Fig1. Graphical abstract
Cytotoxic T-cell infiltration into the tumor microenvironment (TME) correlates with improved clinical outcomes, and immunotherapy aims to harness this immunity. Current strategies include expansion of tumor-infiltrating lymphocytes (TILs), engineered T cells, and checkpoint blockade.
Melanoma is the deadliest form of human skin cancer, with 100,000 new cases annually, and remains a paradigm for cancer immunotherapy. However, despite tumors expressing altered or neoantigens recognized by TILs, 50% of patients develop resistance to treatment. The mechanisms underlying this immune evasion remain incompletely understood.
Melanosomes are large extracellular vesicles (200–500 nm) unique to melanocytes that transport melanin to neighboring cells and serve as key protectors of skin cells against UV-induced DNA damage. Melanosomes differ from exosomes in lineage restriction and cargo composition. Melanoma-derived melanosomes drive fibroblast transformation, lymphangiogenesis, macrophage diversification, and drug resistance. Furthermore, melanosomes promote melanoma resistance by exporting chemotherapeutic drugs. Clinically, melanosome production varies with disease stage, active melanogenesis reduces survival, and melanosome proteins are recognized by T cells in vitiligo patients. However, whether melanosomes directly regulate T-cell function remains unclear.
Memory or naïve CD8+ T cells express T-cell receptors (TCRs) that recognize tumor-associated or neoantigen peptides presented by major histocompatibility complex (MHC) class I molecules. In humans, the human leukocyte antigen (HLA)-A, HLA-B, and HLA-C loci encode the heavy chains of class I MHC, while in mice, the homologs are encoded by the H2K, H2D, and H2L loci.
Our Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| HLA-A-3396H | Recombinant Human HLA-A protein, His-tagged | E.coli | Human | His | 28-303 aa | |
| HLA-A-938H | Recombinant Human HLA-A protein, MYC/DDK-tagged | HEK293 | Human | DDK&Myc |
|
|
| HLA-A-01H | Recombinant Human HLA-A Protein, C-His tagged | HEK293 | Human | His |
|
|
| HLA-B-392H | Recombinant Human HLA-B protein, His/T7-tagged | E.coli | Human | His&T7 | 26-309 a.a. |
|
| HLA-B-1074H | Recombinant Human HLA-B Protein, His (Fc)-Avi-tagged | HEK293 | Human | Avi&Fc&His |
|
|
| HLA-B-242HFL | Recombinant Full Length Human HLA-B Protein, C-Flag-tagged | Mammalian Cells | Human | Flag | Full L. |
|
| HLA-C-268H | Recombinant Human major histocompatibility complex, class I, C, His-tagged | E.coli | Human | His | 25-308 a.a. |
|
| HLA-C-3560HF | Recombinant Full Length Human HLA-C Protein, GST-tagged | In Vitro Cell Free System | Human | GST | Full L. 336 amino acids |
|
T-cell activation involves three signals: Signal 1 is the interaction between TCR and MHC (class I) on antigen-presenting cells or tumor cells; Signal 2 is the interaction between CD28 on T cells and B7-1/B7-2 (also known as CD80 and CD86) on antigen-presenting cells or tumor cells. Once Signal 2 occurs, T cells secrete granzyme B and interleukin-2 (IL-2), collectively termed Signal 3. If the interaction with B7-1/B7-2 is weak or absent, or if PD-1 or CTLA-4 binds B7-1/B7-2 instead of CD28, T cells become tolerant, anergic, or undergo apoptosis.
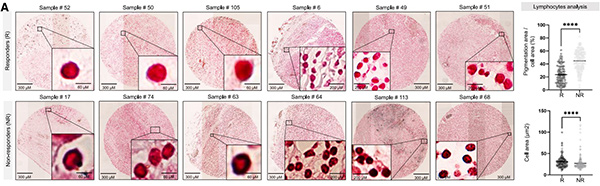
Fig2. The presence of melanosomes correlates with poor prognosis, lack of response to immunotherapy, and decreased T cell cytotoxicity (A) Left: images of melanin-stained FFPE tissue from representative responders (R) and non-responders (NR) melanoma patients. Right: percent of pigmentation area per cell area and cell area (μm2 ).
Our Related Proteins
This study identifies a novel melanoma immune evasion mechanism. Melanosomes bind CD8+ T cells and suppress their activity in a TCR/antigen-dependent manner. Unlike responders, non-responder TILs are surrounded by pigment. In co-culture, melanosomes block interferon (IFN)γ and granzyme B secretion, reducing T-cell cytotoxicity, while inhibiting melanosome release in mice enhances CD8+ infiltration and suppresses tumor growth.
Proteomic, imaging, and immunopeptidomic analyses revealed that melanoma melanosomes are enriched in immune-related proteins and carry HLA molecules, enabling melanoma to secrete HLA that binds TCRs and competes with tumor cells for T-cell recognition. TCR sequencing (TCR-seq) further showed that TILs from non-responders share more clonotypes between melanosomes and melanoma cells compared to responders, indicating direct competition for T-cell binding between melanosomes and tumor cells.
Mechanistically, melanosomes deliver incomplete activation signals, reduce mitochondrial activity, and induce CD8+ T-cell apoptosis. These findings establish melanoma-derived melanosomes as active mediators of immune evasion and potential therapeutic targets.
Related Products & Services
- Immune Checkpoint Proteins
- ADC Target Protein
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- HLA export by melanoma cells decoys cytotoxic T cells to promote immune evasion. Chemla, Yoav et al. Cell, Volume 0, Issue 0

