ADC Target Protein
🧪 CDA002
Source: Human Ascites Fluid
Species: Human
Tag: Non
Conjugation:
Protein Length:
🧪 GPC3-2551H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: 25-559 aa


🧪 F3-28H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length:
$99.00
$198
/ 10μg$284.50
$569
/ 50μg

🧪 EGFR-30H
Source: Human Cells
Species: Human
Tag: His
Conjugation:
Protein Length: 25-645 aa
🧪 ERBB3-44H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: 20-643 aa
🧪 ENG-63H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: 1-586 aa

🧪 EPCAM-81H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: Met1-Lys265
🧪 CD33-459H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length:
🧪 Axl-132M
Source: HEK293
Species: Mouse
Tag: Fc&His
Conjugation:
Protein Length: Met1-Pro443
🧪 CA9-256H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Gln138-Asp414
🧪 Alcam-624M
Source: HEK293
Species: Mouse
Tag: Fc&His
Conjugation:
Protein Length: Met1-Lys527
🧪 TNFRSF8-642H
Source: Human Cells
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: 1-379 a.a.

🧪 EGFR-692H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: 1-640 a.a.
🧪 POSTN-820H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: 1-781 aa
🧪 TACSTD2-887H
Source: HEK293
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: Met1-Thr274
Background
Antibody drug conjugate (ADC) is a new class of cancer therapeutics, which is made by coupling monoclonal antibody (Antibody) and toxic drug small molecule (Payload) through linker. It combines the advantages of highly specific targeting ability and efficient killing effect to achieve accurate and efficient elimination of cancer cells and has become one of the hotspots in anti-cancer drug development.
Structure of ADC drugs
An ADC comprises of three distinct components: the monoclonal antibody (mAb), the cytotoxic drug (also known as payload), and a linker that connects the first two components. The monoclonal antibody is designed to specifically bind to antigens that are overexpressed on the surface of cancer cells. The cytotoxic drug is a highly potent molecule capable of killing cells once internalized. The linker, which can be either cleavable or non-cleavable, functions to sturdily attach the drug to the antibody without compromising the properties of both, and to ensure the controlled release of the cytotoxic drug within the target cell. Thus, the entire ADC structure is precisely engineered to deliver the cytotoxic drug specifically to cancer cells, thereby achieving a targeted therapeutic effect.
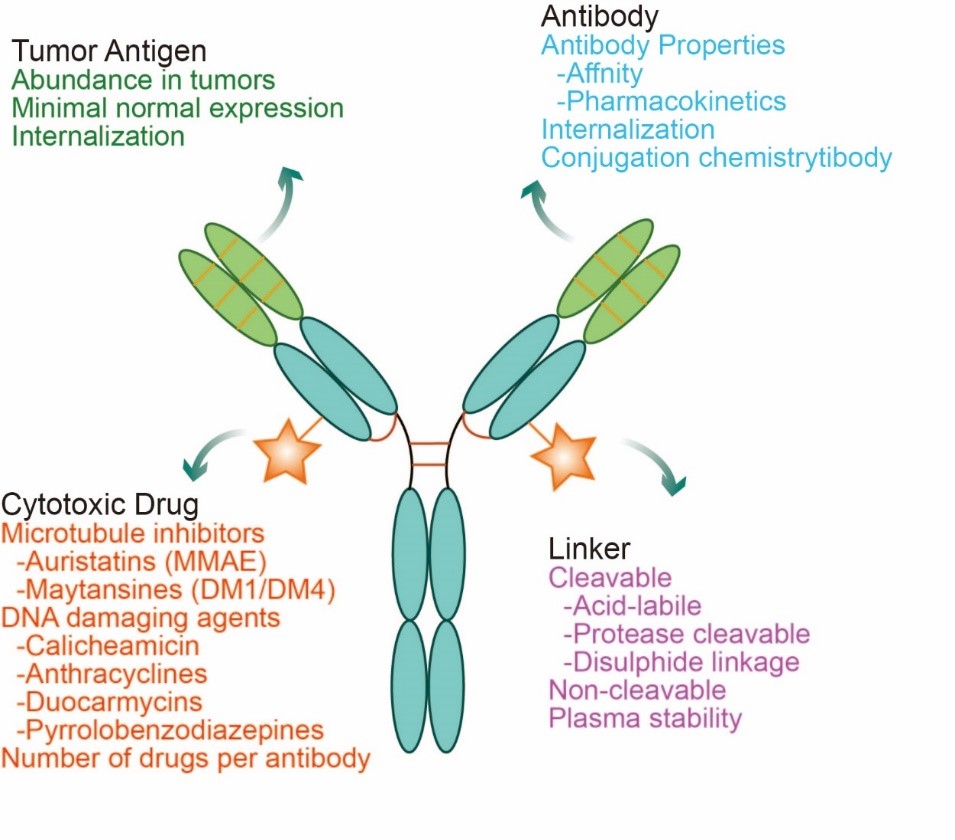
Fig. 1 Structural characterization of ADC drugs
The ideal ADC drug remains stable in the circulation, accurately reaches the therapeutic target, and ultimately releases its cytotoxic payload in the vicinity of the target (e.g., cancer cells). Each of these elements affects the ultimate efficacy and safety of an ADC, and in general the development of an ADC requires consideration of all these key components, including the target antigen, antibody, cytotoxic payload, junction, and choice of coupling method. Antibodies are designed to target specific antigen receptors that are highly expressed in tumor cells, resulting in a "precision-guided" action. ADCs deliver highly selective drugs to tumors, thereby minimizing systemic exposure and improving therapeutic indexes to achieve higher efficacy or fewer side effects.
Mechanism of ADC Drugs
ADCs synergize "specific" targeting and "effective" killing of cancer cells. These drugs act as precision-guided "bio-missiles" with the ability to accurately destroy cancer cells, improve treatment and minimize off-target side effects. Once the mAb of the ADC binds to the target antigen specifically expressed on the cancer cell, the ADC is endocytosed/internalized by the cell to form an early endosome, which then matures into a late endosome and ultimately fuses with the lysosome. The cytotoxic payload ultimately leads to apoptosis or cell death by targeting DNA or microtubules through chemical or enzyme-mediated release in the lysosome. When the released payload is permeable or transmembrane, it may also cause bystander effects to enhance the efficacy of the ADC. In addition, the bystander effect of these drugs may also alter the tumor microenvironment, which in turn may further enhance the killing effect of ADCs.
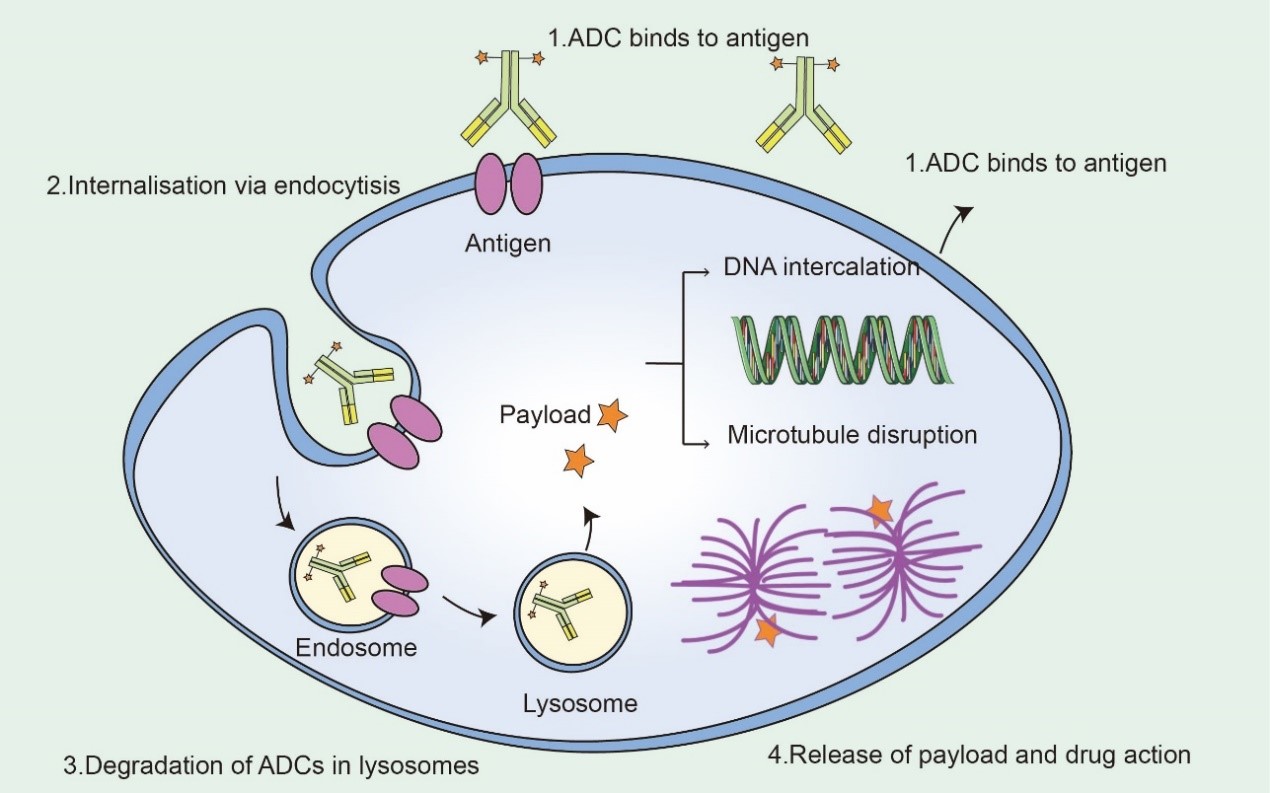
Fig2. Mechanism of ADC Drugs
ADC targets
An antibody-drug conjugate (ADC) is a unique form of medication that leverages the accuracy of antibodies to target specific protein markers within an individual's body. ADCs are specifically structured to maximize the efficacy of drug delivery while minimizing potential harm to non-target cells. This precision is paramount as it ensures increased effectiveness with reduced side effects, qualities highly sought after in pharmacology.
The target proteins serve as biomarkers for the disease state. Their identification often leads to a diagnostic—"overexpression" or abnormal presence of a particular protein may indicate a specific disease or severity therewith. The antibody component of the ADC can seek out these target proteins in a manner comparable to a "guided missile." By binding exclusively to the target protein, the antibody ensures selective delivery of the drug payload to the intended site, keeping healthy, necessary proteins unharmed to maintain overall health and bodily function.
Table1. Target antigens of ADC
|
Target antigens overexpressed in cancer cells |
Target antigens in the tumor vasculature |
|
|---|---|---|
|
Target antigens in the |
||
|
Driver oncogenes |
||
Evolution of ADC drug
From the perspective of drug composition and technical characteristics, ADC drug development can usually be subdivided into three generations. The first generation of ADCs failed due to insufficient toxicity and structural instability prone to off-targeting; the second generation of ADCs failed because of the increased specificity of humanized antibodies and the selection of toxins with higher toxicity, but there was the problem of poor homogeneity due to random coupling, and the third generation of ADCs made the DAR homogeneous and off-targeting toxicity declined with the development of the fixed-point coupling technology.
ADC drug has set off a R&D boom, and the market scale is expanding rapidly. ADC drug R&D has entered a stage of high growth rate in recent years, and several pharmaceutical companies are focusing on the layout in this field. As of the end of June 2023, a total of 15 new ADC drugs have been approved and marketed globally, and the sales volume of some big ADC single products.
Table2. ADCs on the market
|
Targets |
Indications |
Targets |
Indications |
|---|---|---|---|
|
Acute myeloid leukemia |
Triple-negative breast cancer |
||
|
Hodgkin lymphoma |
Head and neck cancer |
||
|
Breast cancer |
Multiple myeloma |
||
|
Acute myeloid leukemia |
Large B-cell lymphoma |
||
|
Hairy cell leukemia |
Cervical cancer |
||
|
Diffuse large B-cell lymphoma |
Gastric or gastroesophageal cancer |
||
|
Diffuse large B-cell lymphoma |
Ovarian Cancer |
||
|
Advanced urothelial cancer |
|
|
Case Study
Case1: LF Larsen, et al. Thrombosis and Hemostasis (ISTH) Congress, 2017
To investigate the influence of different triggers and phospholipids on the thrombin generation response when monitoring therapeutic relevant rFVIIa levels in plasma from haemophilia A (HA) patients.
The researchers find that with nM concentrations of sTF an assay with a significantly improved analytical window and a sensitivity of 1 nM rFVIIa was obtained.
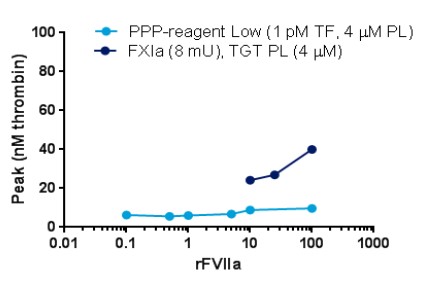
Fig1. Dose-response of rFVIIa with different standard triggers and phospholipids
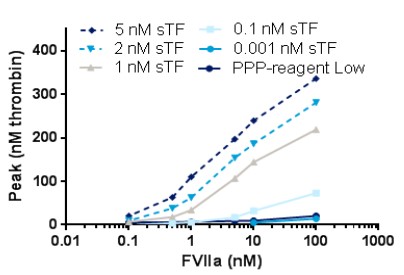
Fig2. Dose-response of rFVIIa when using soluble TF as trigger; PL source is TGT PL (4 µM). PPP-reagent Low is used as comparator
Case2 Anas AR, et al. Mar Drugs. 2015
The blood coagulation cascade involves the human coagulation factors thrombin and an activated factor VII (fVIIa). In this research, researchers explored different strains of toxic Microcystis aeruginosa and cyanobacteria blooms for the probable fVIIa-soluble Tissue Factor (fVIIa-sTF) inhibitors. The fVIIa-sTF active fractions from cultured cyanobacteria and cyanobacteria blooms were subjected to liquid chromatography-mass spectrometry (LC-MS).
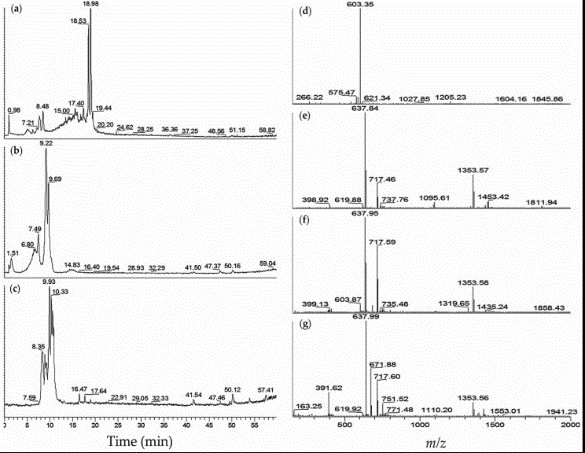
Fig1. LC-MS profiles of M. aeruginosa K139 and NIES-89 cyanobacteria fVIIa-sTF active fractions: (a) Total ion chromatogram (TIC) of M. aeruginosa K139–60% MeOH; (b) TIC of M. aeruginosa NIES-89–40% MeOH; (c) TIC of M. aeruginosa NIES-89–45% MeOH; (d) Mass spectrum (MS) of M. aeruginosa K139–60% MeOH, retention time (tR) 7.3–8.5 min; (e) MS of M. aeruginosa NIES-89–40% MeOH, tR 8.8–9.7 min; (f) MS of M. aeruginosa NIES-89–40% MeOH, tR 6.0–7.9 min; (g) MS of M. aeruginosa NIES-89–45% MeOH, tR 7.9–11.3 min.
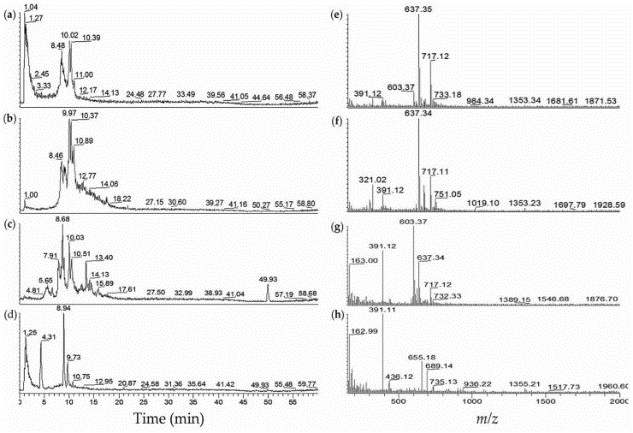
Fig2. LC-MS profiles of fVIIa-sTF active cyanobacterial extracts from algal blooms. (a) Extracted ion chromatogram (EIC m/z 600–800) of JX-1-5 (from Ibaraki)–40% MeOH; (b) EIC m/z 600–800 of JX-1-5–60% MeOH; (c) EIC m/z 600–800 of Koyaike site 2–60% MeOH; (d) EIC m/z 600-800 of Koyaike site 3–40% MeOH; (e) ESI-full MS of JX-1-5–40% MeOH, tR 8.2–10.2 min; (f) ESI-full MS of JX-1-5–60% MeOH, tR 8.1–11.7 min; (g) ESI-full MS of Koyaike site 2–60% MeOH, tR 5.4–14.5 min; (h) ESI-full MS of Koyaike site 3–40% MeOH, tR 0.01–13.7 min.
Case3: Zheng, M., et al. Acta Pharmaceutica Sinica B, 2021
Inhibition of human epidermal growth factor receptor 2 mediated cell signaling pathway is an important therapeutic strategy for HER2-positive cancers. In this study, researchers reported a strategy for the development of artificial antibody based on γ-AApeptides to target HER2 extracellular domain (ECD).
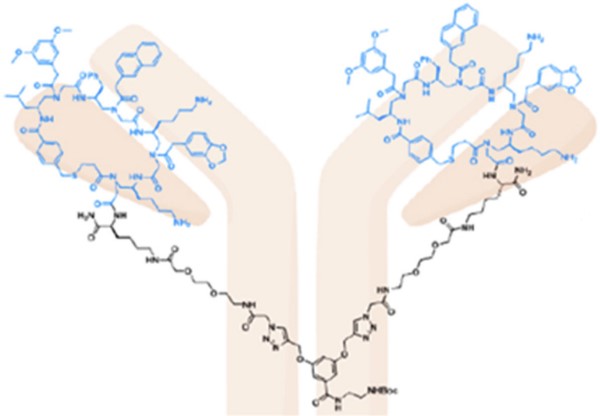
Fig1. Her2 target. γ-AApeptide-based artificial antibody specifically bound to HER2 and antagonized HER2 mediated cell singling both in vitro and in vivo.
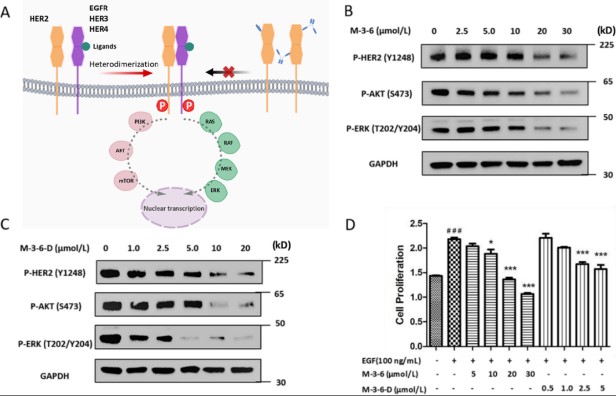
Fig2. (A) Signal transduction pathway mediated by HER2 and proposed inhibition by cyclic peptides monomer and dimer. (B) Western blot analyses of SKBR3 cell lysates following M-3-6 incubation in vitro. M-3-6 treatment resulted in a dose-dependent inhibition of HER2 receptor phosphorylation and a reduction in the phosphorylation of AKT and ERK, downstream signaling of HER2.
Case4: Daniel Nunez, et al. Methods & Clinical Development. 2023
Systemic lupus erythematosus (SLE) is a severe, life-threatening autoimmune disease characterized by widespread immune activation, autoantibodies to self-antigens, and organ dysfunction. As such, therapies that either antagonize or deplete B cells have been investigated in SLE. Although monoclonal antibodies targeting CD19 and CD20 have demonstrated benefit in subsets of patients, relapse is common. Anti-CD19 CAR T cell therapy has been used to treat immunosuppressive refractory SLE patients in the context of a point-of-care program. Initial clinical and correlative data suggest that anti-CD19 CAR T cell therapy is well tolerated and highly effective.
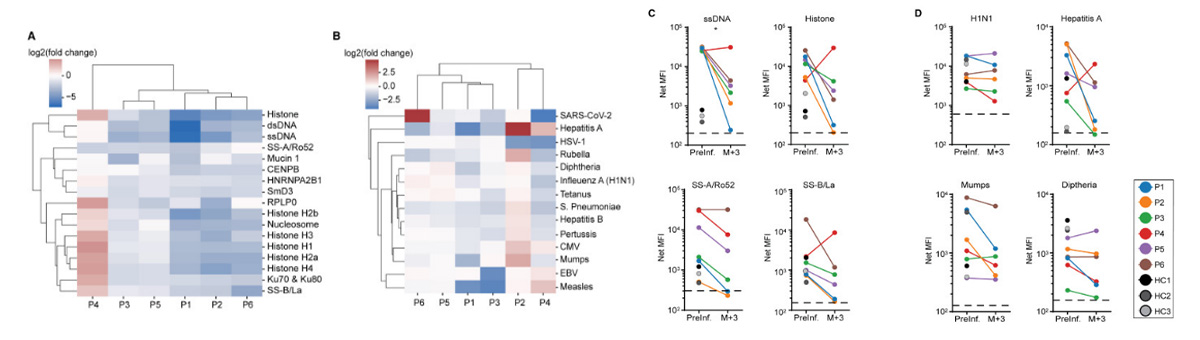
Fig1. Figure 2Effects of anti-CD19 CAR T cell therapy on humoral immunity in SLE patients
(A) SLE-associated and (B) infectious agents or pathogen-associated antibodies prior to and 3 months following anti-CD19 CAR T cell infusion. Antibodies are ordered based on hierarchical clusters formed using the minimum Euclidian distance between all points in each pair of clusters. Values displayed are the log2 transformed ratios of antibody concentration changes from pre-infusion to the third month after infusion (log2 fold change). (C) Quantification of antibodies against single-stranded (ss) DNA, total histone, Sjogren’s syndrome (SS)-A/Ro52, and SS-B/La at baseline and 3 months after CAR T cell infusion (N = 6). (D) Quantification of antibodies against H1N1, hepatitis A, mumps, and diphtheria prior to and 3 months following CAR T cell infusion (N = 6). HC, healthy donor control; MFI, mean fluorescence intensity; P, patient. Dashed black line depicts lower limit of antibody quantification.
Applications
First, the study of ADC targets is also instrumental in understanding drug resistance mechanisms. Overtime, cancer cells may develop resistance to initially effective targeted therapies. By studying ADC targets, researchers can comprehend the molecular basis for such resistance and devise strategies to overcome it, possibly through the modification of the ADC or development of novel ADCs. The study of ADC targets thus paves way for developing ADCs that can overcome drug resistance, thereby improving patient prognosis.
ADC targets study is also crucial for the development of new therapeutic targets. By identifying proteins that are highly expressed on diseased cells but not on healthy ones, novel ADCs can be designed to enhance therapeutic efficacy. Detailed profiling of these potential targets in preclinical models can provide valuable insights into their usefulness for therapeutic intervention. For instance, in non-Hodgkin lymphoma and chronic lymphocytic leukemia, the CD37 antigen is a novel target for ADC therapy.
Moreover, the study of ADC targets can lead to the improvement of pharmacokinetic properties of the ADC, making the drug-delivery more efficient. ADCs with optimized linkers and payloads have shown improved therapeutic indices and decreased toxicity. Thus, a comprehensive understanding of ADC targets can aid researchers in designing ADCs with better pharmacokinetic properties.
Likewise, the ADC targets study can help in bridging the gap between preclinical experiments and clinical trials. Often, promising results in preclinical models may not translate into clinical success due to a lack of understanding of the molecular and cellular aspects of ADC targeting. By deeply analyzing the behavior of ADC targets in different conditions, including the influence of patient-specific factors, researchers can predict the clinical usefulness of specific ADCs more accurately.
Further, studying the ADC targets help in toxicity profiling, providing valuable information about potential off-target effects. This information is crucial for limiting side effects and optimizing dosage regimens.
Advantages
- High Quality: they are manufactured under rigorous quality control standards, ensuring their high quality and reliable performance in your research. These targets are equipped with diverse forms and customized options, catering to your diverse needs for different research purposes.
- Wide Coverage: we provide a rich collection of ADC target proteins, covering both classical targets such as HER2, CD30, and numerous innovative targets, thus facilitating your target discovery and ADC development. Moreover, our ADC target proteins are well-characterized and function-tested to ensure their bioactivity and functionality, thus enabling your studies with solid data.
- Bioactivity: our ADC target proteins possess superior binding efficiency, ensuring optimal delivery of cytotoxic drugs to disease cells, consequently enhancing the therapeutic potential of your ADCs. Finally, we strive to continuously update and stretch our product range to serve your evolving research needs better.
- Technical support: we provide full technical support to assist in product selection, experimental design, protocol optimization, and data interpretation. Our vision is to propel scientific discovery by delivering superior products and solutions for ADC development.
FAQ
-
Q: Are the proteins provided in liquid or lyophilized form?
A: Creative BioMart can provide ADC targets protein either in liquid or lyophilized form.
-
Q: What size of the proteins do you offer?
A: Creative BioMart provides protein in 5ug, 20ug, 50ug, 100ug, even 1mg, different proteins have different size, and you could find more details in the products page or consult us.
-
Q: How do I order a custom ADC target protein?
A: If you don’t find you desired protein in the product list, you could search the protein in the search bar on the top of the website. If you still don’t find the product, or you have a special requirements for the protein, just inquiry us directly.
-
Q: What is the lead time for ADC target proteins?
A: Our products have a lead time of 3 days to 2 weeks. Custom orders have a turnaround time of 6-8 weeks. Contact us for more information.


-cover.jpg)