Uncategorized Thursday, 2026/01/22
Scientists from Case Western Reserve University and other institutions have discovered a key enzyme called SCoR2 that acts as a "master switch" determining the fate of fat.
Have you ever wondered why some people "gain weight just by drinking water" while others seem naturally "thin"? The battle with fat may be orchestrated inside cells by a key enzyme. Obesity and metabolic dysfunction–associated steatotic liver disease (MASLD) have become global health crises affecting the quality of life for hundreds of millions, while traditional weight-loss strategies often face challenges of rebound and metabolic side effects.
Obesity Targets
Find More Targets on Our Website
Recently, a research team from Case Western Reserve University and other institutions published a study in Science Signaling revealing that an enzyme called SCoR2 serves as the "master switch" for fat fate. Targeting and inhibiting it can simultaneously block fat synthesis and promote fat burning, offering a novel direction for treating obesity and fatty liver disease.
To understand this discovery, we must first know the body's "gaseous messenger"—nitric oxide (NO). Not just a component of car exhaust, NO is a crucial signaling molecule that regulates metabolism by adding S-nitrosylation modifications to proteins (like putting a "hat" on them). Putting on and taking off this "hat" finely regulates metabolic and physiological processes.
While sirtuins were previously known to regulate metabolism by removing acetyl groups from proteins, this new study reveals that SCoR2 is a "denitrosylase"—an enzyme specifically responsible for removing the NO "hat" from proteins. When SCoR2 is active, it continuously removes the "hat" from key regulatory proteins in adipose tissue and the liver, directly activating fat synthesis and storage programs while inhibiting fat burning.
To verify SCoR2's role, the researchers conducted a series of rigorous experiments. They first discovered that SCoR2 protein levels in mice directly and positively correlated with body weight. Subsequently, through genetic knockout or pharmacological inhibition of SCoR2 activity, the results were striking: mice lacking SCoR2 function effectively resisted weight gain even when fed a high-fat diet and were also protected against fatty liver. These mice's livers favored oxidative fat burning, white adipose tissue expansion was significantly reduced, and the drug simultaneously lowered harmful cholesterol levels.
The researchers explained that the NO "hat" in the liver inhibits proteins involved in fat and cholesterol synthesis, while in adipose tissue it inhibits the genetic switches of the fat synthesis program. SCoR2's continuous "de-hatting" is equivalent to constantly flooring the accelerator on fat synthesis; inhibiting SCoR2 is like taking your foot off that accelerator.
SCoR2 synergistically promotes obesity and fatty liver through different targets in adipose tissue and the liver. In adipocytes, SCoR2 deletion keeps myosin 9 protein S-nitrosylated ("hatted"), inhibiting the activity of pro-lipogenic transcription factors like PPARγ and SREBP1 and preventing excessive fat storage. In hepatocytes, inhibiting SCoR2 keeps fat synthesis–related enzymes "hatted," directly reducing new fat synthesis while inducing oxidative fat breakdown. This dual-organ, dual-regulation mechanism makes SCoR2 a highly attractive therapeutic target.
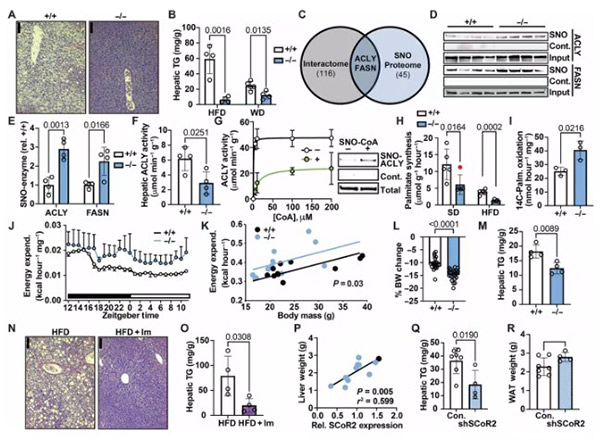
Fig1: Inhibition of lipid synthesis and enhancement of fat oxidation through S-nitrosylation; deletion or pharmacological inhibition of hepatic SCoR2 prevents steatosis.
The study's strength comes not only from animal experiments but also from human sample data corroborating the link between SCoR2 and obesity/fatty liver. The researchers found that an obesity-associated genetic polymorphism increases SCoR2 mRNA expression. In adipose and liver tissues from obese and fatty liver patients, SCoR2 protein or mRNA abundance directly and positively correlated with adipocyte size and degree of liver steatosis. This indicates that SCoR2 also plays a key driver role in the development of human metabolic diseases.
The researchers stated they have a new class of drugs that can prevent weight gain and lower cholesterol. This potential therapy also benefits the liver, and they are currently advancing it to clinical trials, which are expected to take approximately 18 months. Translation efforts will be supported by the Harrington Discovery Institute. This study not only identifies a new enzyme but also reveals a novel metabolic regulatory axis driven by protein S-nitrosylation modification. If drugs targeting SCoR2 can safely replicate the animal results in human trials, it means we may possess a weapon to "reset" the fat metabolism bias from its root cause—bringing a revolutionary breakthrough for treating obesity and related metabolic diseases!
Related Products & Services
- Obesity Targets
- Enzyme Activity Assay
- Computer-aided Enzyme Design
- Enzyme Target and Screening
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Venetos NM, Stomberski CT, Zhou HL, Qian Z, McLaughlin PJ, Bansal PK, Feczko J, Bederman I, Nguyen H, Hausladen A, Schindler JC, Grimmett ZW, Brunengraber H, Premont RT, Stamler JS. The protein denitrosylase SCoR2 regulates lipogenesis and fat storage. Sci Signal. 2025 Dec 23;18(918):eadv0660. doi: 10.1126/scisignal.adv0660. Epub 2025 Dec 23. PMID: 41433418.
