Uncategorized Thursday, 2026/01/22
Melanoma cells secrete melanosomes that carry major histocompatibility complex molecules and tumor antigens. By binding to the T-cell receptors of CD8⁺ T cells, these melanosomes induce dysfunction and apoptosis, facilitating tumor immune evasion. Inhibiting melanosome secretion can significantly enhance anti-tumor immune effects.
Among skin cancers, melanoma poses a deadly threat with 100,000 new cases annually. While immunotherapy offers hope for some patients, nearly half do not respond to treatment. Even when tumors express mutant antigens recognizable by immune cells, the CD8⁺ T cells responsible for tumor killing struggle to function effectively.
Recently, a groundbreaking study published in Cell has unveiled melanoma's stealth tactic: melanosomes secreted by tumor cells can transform into "immune traps" that incapacitate CD8⁺ T cells that should be killing the tumor. This discovery provides a novel direction for overcoming immunotherapy resistance.
Presence of Melanosomes Directly Correlates with Treatment Efficacy and Prognosis
By analyzing The Cancer Genome Atlas (TCGA) database and 69 metastatic melanoma patient samples, the study found that patients with high expression of melanosome-related genes had higher recurrence risk and shorter survival. Non-responders to immunotherapy had significantly more pigment-loaded T cells than responders, and these T cells were smaller in size, exhibiting typical functional exhaustion characteristics.
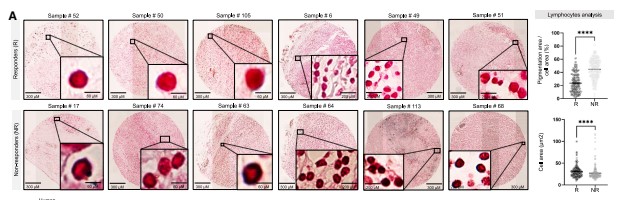
Figure 1: Presence of melanosomes correlates with poor prognosis, immunotherapy non-response, and reduced T cell cytotoxicity.
Further experiments confirmed that melanosomes bind to CD8⁺ T cells with extremely high efficiency, completing binding within 30 minutes and maintaining stable association for over four hours. A single melanoma cell can secrete approximately 40 melanosomes per day. When tumor-infiltrating lymphocytes were pre-exposed to melanosomes, their secretion of interferon-γ and granzyme B was substantially reduced, and their ability to kill tumor cells significantly decreased.
Our related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| IFNG-510H |
Active Recombinant Human IFNG
|
HEK293 | Human | Non | 24-166 a.a. | |
| IFNG-14081H | Recombinant Human IFNG, His-tagged | E.coli | Human | His | 22-166a.a. | |
| IFNG-4340F | Recombinant Ferret IFNG Protein | Yeast | Ferret | Non | 164aa | |
| IFNG-1165C |
Active Recombinant Cynomolgus IFNG Protein
|
E.coli | Cynomolgus | Non | 24-165 a.a. |
|
| GZMB-296H |
Active Recombinant Full Length Human GZMB, His tagged
|
HEK293 | Human | His | Full L. 1-247 aa | |
| GZMB-33H |
Active Recombinant Human GZMB Protein (19-247aa), C-His tagged
|
Insect Cells | Human | His | 19-247 aa | |
| Gzmb-942M | Recombinant Murine Granzyme B | Insect Cells | Mouse | Non | ||
| Gzmb-10561M | Recombinant Mouse Gzmb Protein, His (Fc)-Avi-tagged | HEK293 | Mouse | Avi&Fc&His | ||
| GZMB-3094H | Recombinant Human GZMB Protein, His (Fc)-Avi-tagged | HEK293 | Human | Avi&Fc&His | ||
| GZMB-4026M | Recombinant Mouse GZMB Protein, His (Fc)-Avi-tagged | HEK293 | Mouse | Avi&Fc&His | ||
| HLA-A-3396H | Recombinant Human HLA-A protein, His-tagged | E.coli | Human | His | 28-303 aa | |
| HLA-A-938H | Recombinant Human HLA-A protein, MYC/DDK-tagged | HEK293 | Human | DDK&Myc |
|
|
| ZAP70-3777H | Recombinant Human ZAP70, GST-tagged | E.coli | Human | GST | 235-393aa |
Inhibiting Melanosome Secretion Can Break the Tumor Growth Spell
To verify the role of melanosomes, researchers used the depigmenting agent kojic acid to inhibit tyrosinase activity, thereby reducing melanosome secretion. Experiments showed that kojic acid did not affect tumor cell proliferation or CD8⁺ T cell survival, yet specifically reduced melanoma pigmentation and melanosome release.
In mouse melanoma models, kojic acid treatment significantly restricted tumor growth, prolonged mouse survival, and substantially increased CD8⁺ T cell infiltration in tumor tissues. When CD8⁺ T cells were depleted using antibodies, the anti-tumor effect of kojic acid was diminished, confirming that its action depends on CD8⁺ T cells. Additionally, melanoma cells with tyrosinase knocked out via CRISPR-Cas9 displayed a similar anti-tumor phenotype, further validating the critical role of melanosomes in tumor progression.
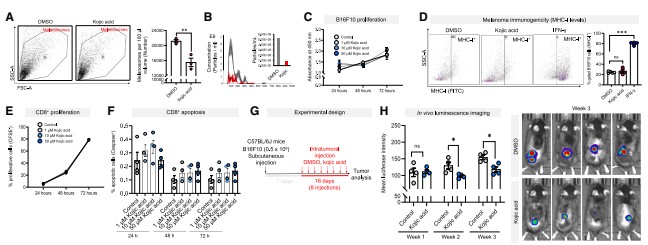
Figure 2: Inhibiting melanosome secretion from melanoma cells blocks tumor progression and induces CD8⁺ T cell infiltration.
MHC-TCR Binding: The Core Mechanism of Melanosome "Capture" of T Cells
Proteomic analysis revealed that, unlike exosomes, melanoma-derived melanosomes are enriched in immune-related proteins, particularly MHC class I molecules (HLA molecules in humans) . These MHC class I molecules can properly load tumor antigen peptides and possess the ability to bind T-cell receptors (TCRs).
Electron microscopy observation revealed that melanosomes form membrane-to-membrane contacts with CD8⁺ T cells resembling immune synapses, and this binding is antigen-specific—only melanosomes carrying the corresponding antigen can bind CD8⁺ T cells expressing the specific TCR. When melanosomes lacked MHC class I molecules, their interaction with CD8⁺ T cells was significantly diminished, proving that MHC-peptide-TCR interaction is the core mechanism of binding.
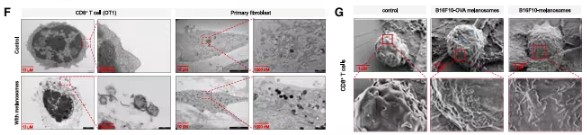
Figure 3: MHC class I molecules on the melanosome membrane bind to T-cell receptors on CD8⁺ T cells.
Melanosomes Carry Highly Immunogenic Antigens and "Compete" with Tumor Cells for T Cells
Immunopeptidomic analysis showed that MHC-binding peptides carried by melanosomes had 83.8% overlap with peptides on tumor cell surfaces, but the peptides on melanosomes had higher MHC binding affinity and hydrophobicity, making them more immunogenic. The study identified a total of 25 tumor-associated antigens in melanosomes, with expression levels significantly higher than in tumor cells, while also discovering 3 mutation-derived neoantigens.
TCR sequencing results indicated that melanosome-bound CD8⁺ T cells and tumor cell-bound CD8⁺ T cells share numerous TCR clonotypes. In immunotherapy non-responders, the proportion of tumor cell-specific TCR clones was significantly reduced, suggesting that melanosomes compete with tumor cells for CD8⁺ T cell binding, acting as a physical barrier between T cells and tumor cells.
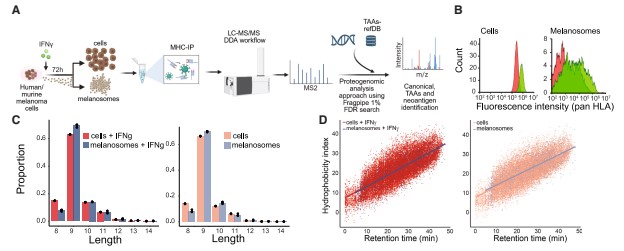
Figure 4: MHC immunopeptidome analysis of human melanoma melanosomes reveals that melanosomes carry tumor-associated antigens and neoantigens.
Melanosomes Induce T Cell "Dysfunction" and Apoptosis
Single-cell RNA sequencing revealed that melanosome-bound CD8⁺ T cells display a unique transcriptional signature: upregulation of anti-apoptotic, adhesion, and inflammation-related genes, and downregulation of proliferation-related genes. Although interferon-γ mRNA levels were slightly elevated in these cells, the TCR signaling pathway was significantly suppressed, ZAP70 phosphorylation (a marker of TCR activation) was absent, mitochondrial function declined, presenting a "dysfunctional" state.
Ultimately, melanosome binding leads to increased apoptosis of CD8⁺ T cells without inducing expression of classic exhaustion markers such as PD1 and TIM3, indicating this is a novel T cell function suppression mechanism.
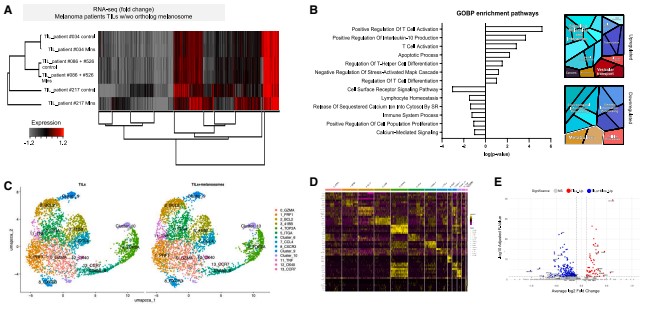
Figure 5: Melanosome binding to CD8⁺ T cells induces a suboptimal transcriptional profile, reduces T-cell receptor signaling and mitochondrial activity, and leads to apoptosis.
In Summary, this study is the first to comprehensively reveal a novel mechanism by which melanoma achieves immune evasion through melanosomes: melanosomes act as "immune decoys" secreted by tumor cells, carrying MHC molecules and highly immunogenic antigens that bind to CD8⁺ T cell TCRs, induce their dysfunction and apoptosis, thereby helping tumors evade immune system attack.
This discovery provides a new therapeutic target for melanoma treatment. Inhibiting melanosome secretion with drugs such as kojic acid, or developing inhibitors targeting pathways involved in melanosome formation and secretion, holds promise for overcoming immunotherapy resistance and enhancing the anti-tumor activity of CD8⁺ T cells.
In the future, combining such drugs with immune checkpoint inhibitors or adoptive T cell therapy may bring curative hope to more melanoma patients.
With deeper exploration of the mechanisms underlying melanosome-mediated immune evasion, we hope to develop more precise and effective treatment strategies, making once "stubborn" melanoma no longer difficult to treat, and injecting new vitality into the field of cancer immunotherapy.
Related Products & Services
- Cell and Gene Therapy
- Immune Checkpoint Proteins
- ADC Target Protein
- PROTAC Targets
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Chemla Y, Itzhaki O, Melamed S, et al. HLA export by melanoma cells decoys cytotoxic T cells to promote immune evasion. Cell. Published online December 15, 2025. doi:10.1016/j.cell.2025.11.020
