Uncategorized Sunday, 2025/09/07
Glycosphingolipid Synthesis is the Key! This study clarifies for the first time that glucose-dependent glycosphingolipid synthesis serves as a "metabolic checkpoint" for CD8⁺ T cells to exert their anti-cancer functions. When this pathway is blocked, T cells face the dilemma of being "armed but without ammunition"—able to infiltrate tumors yet incapable of effectively killing cancer cells.
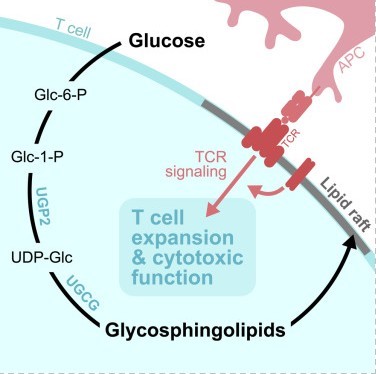
Fig1. Graphical abstract
The rise of tumor immunotherapy has brought new hope to cancer patients, but most still face the challenge of insufficient efficacy. Among them, CD8⁺ T cells, as the "main force" against tumors, have their function finely regulated by metabolic processes. The latest research reveals that glucose not only provides energy to these cells but also empowers their anti-cancer capabilities through a previously overlooked pathway.
In a study published in the journal Cell Metabolism, titled "Glucose-dependent glycosphingolipid biosynthesis fuels CD8⁺ T cell function and tumor control," a team from the Van Andel Institute discovered that glycosphingolipids (GSL) produced through glucose metabolism are the "core fuel" for CD8⁺ T cells to maintain their cytotoxic function. This finding provides a new target for enhancing the efficacy of tumor immunotherapy.
Previously, it was believed that glucose in CD8⁺ T cells primarily generated energy and biosynthetic precursors through glycolysis. However, using ¹³C stable isotope tracing, this study revealed another crucial pathway: in physiologically activated CD8⁺ effector T cells, glucose is largely converted into uridine diphosphate glucose (UDP-Glc), which is a core precursor for glycosphingolipid (GSL) synthesis.
Experimental results showed that in a Listeria-infected mouse model, approximately 20% of GSL molecules in CD8⁺ effector T cells were labeled with ¹³C just two hours after ¹³C-glucose infusion, and the total amount of glycosphingolipids was 3-4 times that of resting T cells. Hexosylceramide (HexCer) and GA1 ganglioside had the highest labeling rates, suggesting rapid synthesis and turnover of these lipids in activated T cells. Surprisingly, human CD8⁺ T cells, when activated in vitro, also directed glucose towards the synthesis of glycosphingolipids like GM3, indicating that this mechanism is conserved across species.
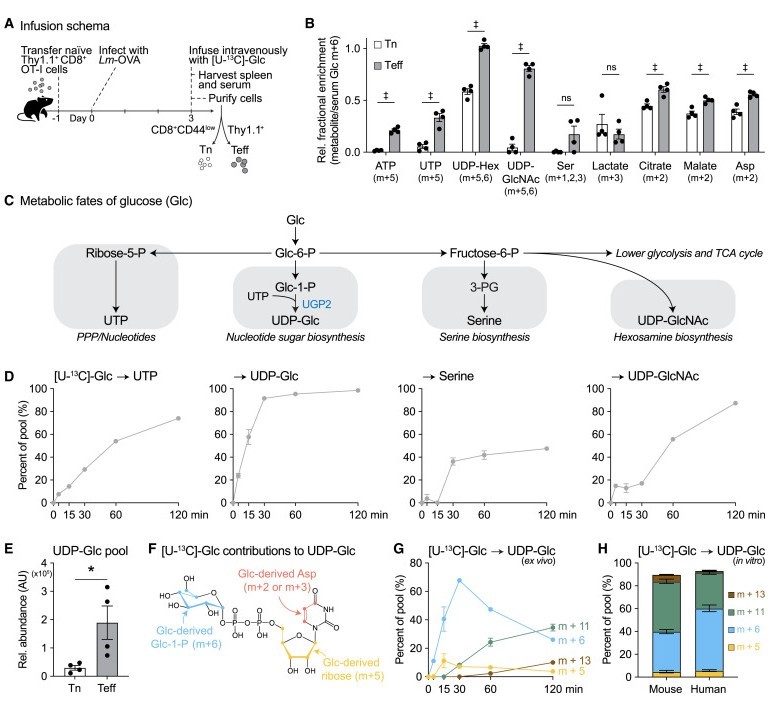
Fig2. UDP-Glc biosynthesis is a major metabolic fate of glucose in physiologically activated CD8+ T cells
To confirm the role of glycosphingolipids, the research team blocked their synthesis through genetic editing and drug intervention:
UGCG Gene Knockout: UGCG is a key enzyme in glycosphingolipid synthesis. Its knockout reduced HexCer and GA1 content in CD8⁺ T cells by over 70%, decreased in vitro proliferation capacity by 40%, and the number of antigen-specific T cells reduced by 2.5 times in a Listeria infection model.
Drug Inhibition: Treating T cells with eliglustat (a UGCG inhibitor) led to a dose-dependent decrease in glycosphingolipid synthesis. At a concentration of 4μM, T cell proliferation rate decreased by 50%, and the expression of cytotoxic marker granzyme B (GZMB) decreased by 60%.
In Vivo Anti-Tumor Experiment: Adoptively transferring UGCG-deficient CD8⁺ T cells into melanoma model mice resulted in the tumor growing at twice the rate of the control group, reducing mouse survival by 40%, while the proportion of GZMB positive cells among tumor-infiltrating T cells decreased by 55%.
Further research revealed that the core function of glycosphingolipids is to maintain the integrity of lipid rafts on the cell membrane—a "signaling platform" for T cell receptor (TCR) signal transduction. In UGCG-deficient T cells, the binding capacity of the lipid raft marker cholera toxin B subunit (CTxB) decreased by 40%. Upon TCR activation, the phosphorylation levels of PLCγ1 and c-Jun decreased by 30%-50%, leading to inhibited synthesis of downstream cytotoxic molecules like granzyme and perforin.
Targeting glycosphingolipid synthesis may enhance anti-Cancer ability.
This study clarifies for the first time that the glucose-dependent glycosphingolipid synthesis is a "metabolic checkpoint" for CD8⁺ T cells to exert their anti-cancer function. When this pathway is blocked, T cells are left in an "armed but without ammunition" situation—able to infiltrate tumors but unable to effectively kill cancer cells.
This finding provides a new perspective for tumor immunotherapy: by regulating glycosphingolipid synthesis (such as supplementing UDP-Glc precursors or activating UGCG activity), the cytotoxic function of CD8⁺ T cells can be enhanced. Meanwhile, the study also suggests caution in using glycosphingolipid synthesis inhibitors (like eliglustat) as they may inadvertently "harm" anti-cancer T cells.
In the future, exploring how to "replenish" glycosphingolipid synthesis precursors for CD8⁺ T cells within the tumor microenvironment may become a key strategy to enhance the efficacy of immune checkpoint inhibitors.
Related Products & Services
- Cytokines for Organoid Culture
- ADC Target Protein
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Immune Checkpoint Proteins
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Joseph Longo,Lisa M. DeCamp,Brandon M. Oswald, et al. Glucose-dependent glycosphingolipid biosynthesis fuels CD8+ T cell function and tumor control, Cell Metabolism (2025). DOI:10.1016/j.cmet.2025.07.006
