Cytokines for Organoid Culture

🧪 BMP2-01H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 283-396 aa
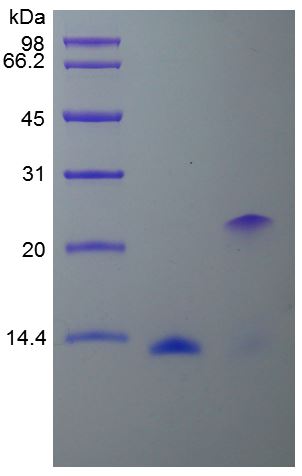

🧪 EGF-04H
Source: E.coli
Species: Human
Tag:
Conjugation:
Protein Length:
$149.00
$298
/ 1mg

🧪 FGF2-11H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length:
$199.00
$398
/ 100μg$699.00
$1,398
/ 1mg

🧪 IGF1-06H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 70
🧪 IGF-08H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length:
$119.00
$238
/ 100 µg
🧪 BMP7-10H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length:
🧪 BMP2-16H
Source: HEK293
Species: Human/Mouse/Rat/Rhesus/Canine
Tag: Fc
Conjugation:
Protein Length: Gln283-Arg396
🧪 FGF9-202H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: 4-208 a.a.
🧪 NOG-817H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: 1-232 a.a.
🧪 RTN4R-840H
Source: HEK293
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: Met1-Ser447
🧪 Rtn4r-2301M
Source: HEK293
Species: Mouse
Tag: His
Conjugation:
Protein Length: Met1-Ser447
🧪 Rtn4r-2306M
Source: HEK293
Species: Mouse
Tag: Fc&His
Conjugation:
Protein Length: Met1-Ser447
🧪 NOG-3156H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Met1-Cys232

🧪 EGF-556H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length:
.jpg)
🧪 Hgf-8665M
Source: HEK293
Species: Mouse
Tag: Non
Conjugation:
Protein Length: 1-728 a.a.
Background
Organoid cultures typically contain multiple components and can be highly variable for different types of organoids. This is achieved by optimizing culture growth conditions. To satisfy your requirements, Creative BioMart has developed a series of high-quality cytokines for organoid culture that have been verified to promote organoid growth.
What is organoids?
Organoids are in vitro miniaturized and simplified model systems of organs that have gained enormous interest for modeling tissue development and disease, and for personalized medicine, drug screening, and cell therapy. Organoids are derived from either pluripotent or tissue-resident stem (embryonic or adult) or progenitor or differentiated cells from healthy or diseased tissues, such as tumors.
Organoids can be established for an increasing variety of organs, including but not limited to the gut, stomach, kidney, liver, pancreas, mammary glands, prostate, upper and lower airways, thyroid, retina, and brain.
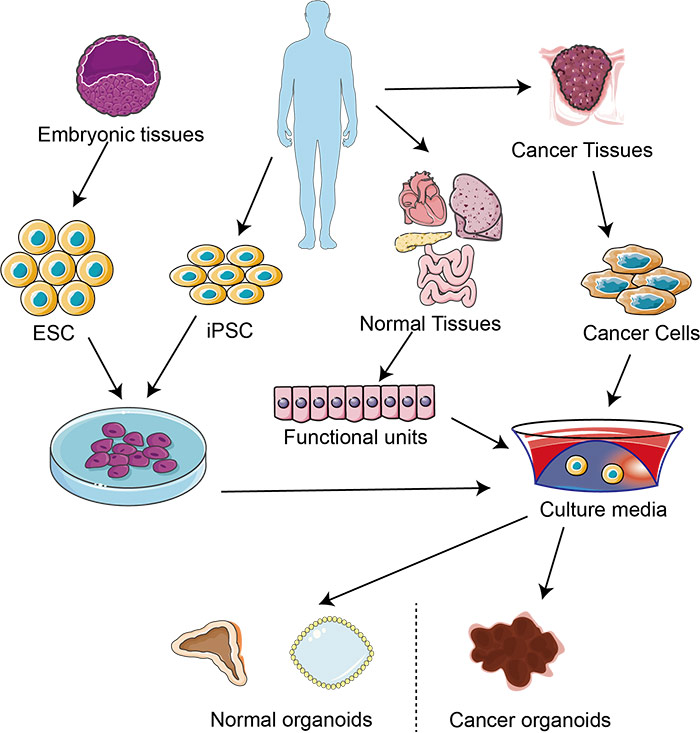
Fig1. 3D culture system
Organoid Culture Is Challenging
Organoid research intertwined with 3D cell culture, stem cell, and tissue engineering. Self-organization within the organoid occurs through spatially restricted lineage commitment and cell sorting, which requires activation of various signaling pathways mediated by intrinsic cellular components or extrinsic environments such as extracellular matrix (ECM) and media.
Organoids are derived from stem cells, and cultured in particular culture media to ensure appropriate organoid growth and development. Organoid cultures typically contain multiple components and can be highly variable for different types of organoids. Identifying and optimizing the conditions for culturing organoids can be technically challenging. This is achieved by optimizing culture growth conditions, such as providing a basement membrane matrix and adding a selection of agonists (e.g. Wnt and tyrosine kinase receptor) and inhibitors (e.g. BMPs/TGFB).
Why Choose Creative BioMart?
Creative BioMart is a professional protein supplier for organoid research. To satisfy your requirements, we have developed a series of high-quality cytokines for organoid culture that have been verified to promote organoid growth.
- Verified Bioactivity
- Batch-to-batch Consistency
- Low Endotoxin
- High Yield
- High Purity
- Animal-free
- High Stability
- Low Immunogenicity
- Vast Selection
Cytokines for Organoid Culture
|
Organoid Type |
Cytokines |
|---|---|
|
Gastric epithelial |
EGF, NOG/Noggin, RSPO1/ R-spondin 1, WNT3A, FGF10, Gastrin, Activin A, FGF4, BMP4 |
|
Intestinal epithelial |
EGF, NOG/Noggin, RSPO1/ R-spondin 1, WNT3A, Activin A, FGF4, BMP4, IGF1, FGF2 |
|
Liver |
EGF, NOG/Noggin, RSPO1/ R-spondin 1, WNT3A, Activin A, FGF2, FGF4, BMP4, BMP2, HGF, FGF7, TGFA, Insulin |
|
Lung |
NOG/Noggin, RSPO1/ R-spondin 1, Activin A, FGF2, FGF7, FGF4, BMP4, FGF10 |
|
Brain |
|
|
Kidney |
|
|
Pancreas |
|
|
Prostate gland |
EGF, NOG/Noggin, RSPO1/ R-spondin 1, WNT10B, Activin A, FGF2, FGF10 |
|
Breast |
EGF, NOG/Noggin, RSPO1/ R-spondin 1, RSPO2, FGF2, FGF10, WNT3A, NGR1 |
|
Retina |
|
|
Inner ear |
Featured Cytokines (Partial List)
|
Cat.No. |
Product Name |
|---|---|
|
Activin A-01HG |
|
|
BMP4-35HG |
Active Recombinant Human BMP4 Protein (Lys303-Arg408), GMP Grade, Animal-Free |
|
BMP7-016H |
|
|
DNF-243H |
|
|
EGF-12320H |
|
|
EGF-832HG |
|
|
FGF10-4097H |
|
|
FGF10-81H |
|
|
FGF2-33HG |
Active Recombinant Human FGF2 Protein (Pro143-Ser288), GMP Grade, Animal-Free |
|
FGF4-038H |
|
|
FGF7-142HG |
|
|
FGF7-4837HF |
|
|
FGF8-01H |
|
|
FGF9-202H |
|
|
GAST-13165H |
|
|
GDNF-01H |
|
|
GF2-001H |
|
|
HGF-143HG |
|
|
HGF-18H |
|
|
IGF-08H |
|
|
IGF1-126H |
|
|
INS-315H |
|
|
MP2-01H |
|
|
NOG-817H |
|
|
NRG1-7321H |
|
|
RSPO1-351H |
|
|
RSPO2-2454H |
|
|
SHH-3963H |
|
|
TGFA-3032H |
|
|
TGFB1-3209H |
|
|
WNT10B-112H |
|
|
WNT3A-1700H |
Case Study
Case 1: Wang Z, McWilliams-Koeppen HP, Reza H, et al. 3D-organoid culture supports differentiation of human CAR+ iPSCs into highly functional CAR T cells. Cell Stem Cell. 2022 Apr 7;29(4):515-527.e8. doi: 10.1016/j.stem.2022.02.009. Epub 2022 Mar 11. Erratum in: Cell Stem Cell. 2022 Apr 7;29(4):651-653. PMID: 35278370; PMCID: PMC9119152.
Unlimited generation of chimeric antigen receptor (CAR) T cells from human-induced pluripotent stem cells (iPSCs) is an attractive approach for "off-the-shelf" CAR T-cell immunotherapy. Approaches to efficiently differentiate iPSCs into canonical αβ T cell lineages, while maintaining CAR expression and functionality, however, have been challenging. The authors report that iPSCs reprogramed from CD62L+ naive and memory T cells followed by CD19-CAR engineering and 3D-organoid system differentiation confers products with conventional CD8αβ-positive CAR T cell characteristics. Their study establishes feasible methodologies to generate highly functional CAR T cells from iPSCs to support the development of "off-the-shelf" manufacturing strategies.
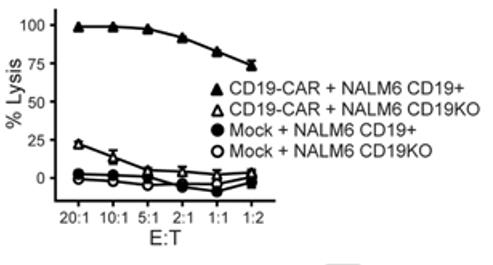
Fig1. Cytotoxic activity of iPSC CD19-CAR T cells against CD19+ or CD19-negative/knockout (CD19KO) NALM6
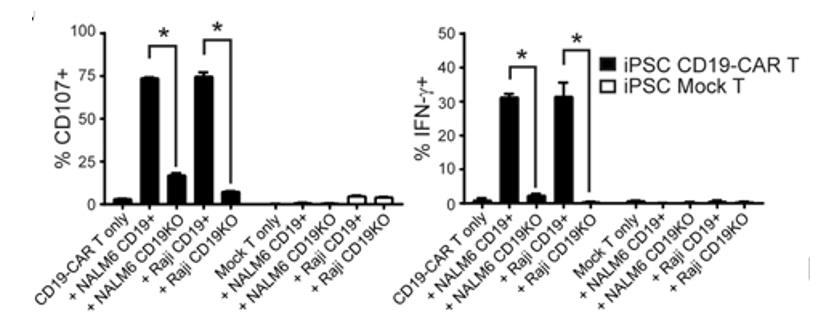
Fig2. Degranulation (i.e., surface CD107, left) and intracellular IFN-g levels (right) in iPSC-derived mock-transduced (Mock) or CD19-CAR T cells was measured by flow cytometry after co-culture with the indicated stimulator cells (X-axis labels) at an E:T ratio of 1:1 for 5 hours in the presence of the Golgi Stop protein transport inhibitor. *, P < 0.01 by Students t-test.
Case 2: Lindemans CA, Calafiore M, Mertelsmann AM, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015 Dec 24;528(7583):560-564. doi: 10.1038/nature16460. Epub 2015 Dec 9. PMID: 26649819; PMCID: PMC4720437.
Using ex vivo organoid cultures, here the researchers show that innate lymphoid cells (ILCs), potent producers of interleukin-22 (IL-22) after intestinal injury, increase the growth of mouse small intestine organoids in an IL-22-dependent fashion. Recombinant IL-22 directly targeted ISCs, augmenting the growth of both mouse and human intestinal organoids, increasing proliferation and promoting ISC expansion. IL-22 induced STAT3 phosphorylation in Lgr5(+) ISCs, and STAT3 was crucial for both organoid formation and IL-22-mediated regeneration.
ATOH1-deficient organoid culture demonstrated that IL-22 induced epithelial regeneration independently of the Paneth cell niche. Their findings reveal a fundamental mechanism by which the immune system is able to support the intestinal epithelium, activating ISCs to promote regeneration.
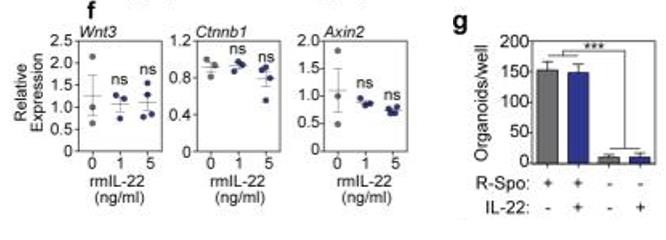
Fig1. f, RT qPCR of relative mRNA expression of Wnt3, Ctnnb1, and Axin2 genes of the Wnt/β-catenin axis in SI organoids cultured +/− rmIL-22; n=3 (0-1ng/ml) and n=4 (5ng/ml) mice/group. g, Numbers of SI organoids/well +/− rmIL-22 (5ng/ml) in the presence or absence of R-spondin-1 (n=6 wells/group).
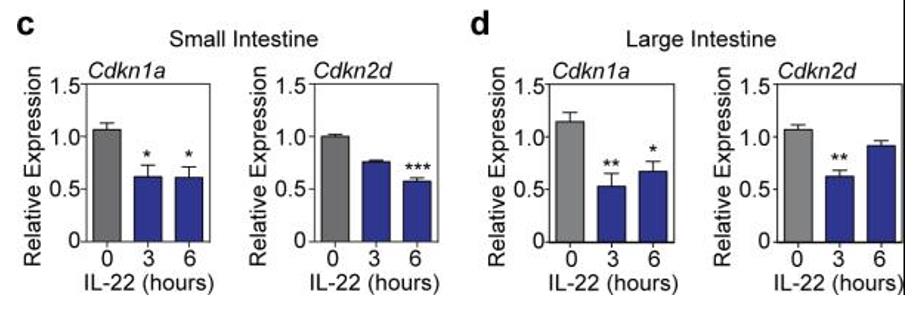
Fig2. Cdkn1a (p21) and Cdkn2d (p19) mRNA expression (RT qPCR) in organoids cultured from (c) SI and (d) LI crypts for 24 hours with 0, 3, or 6 hours exposure to IL-22 prior to harvesting; Kruskal-Wallis analysis, n=6 mice/group combined from two independent experiments.
Case 3: Kakni P, Truckenmüller R, Habibović P, van Griensven M, Giselbrecht S. A Microwell-Based Intestinal Organoid-Macrophage Co-Culture System to Study Intestinal Inflammation. Int J Mol Sci. 2022 Dec 6;23(23):15364. doi: 10.3390/ijms232315364. PMID: 36499691; PMCID: PMC9736416.
Macrophages are crucial for maintaining intestinal homeostasis, but they also play a central role in chronic pathologies of the digestive system. The authors developed a versatile microwell-based intestinal organoid-macrophage co-culture system that enables us to recapitulate features of intestinal inflammation. This microwell-based platform facilitates the controlled positioning of cells in different configurations, continuous in situ monitoring of cell interactions, and high-throughput downstream applications. Using this novel system, we compared the inflammatory response when intestinal organoids were co-cultured with macrophages versus when intestinal organoids were treated with the pro-inflammatory cytokine TNF-α. Furthermore, they demonstrated that the tissue-specific response differs according to the physical distance between the organoids and the macrophages and that the intestinal organoids show an immunomodulatory competence.
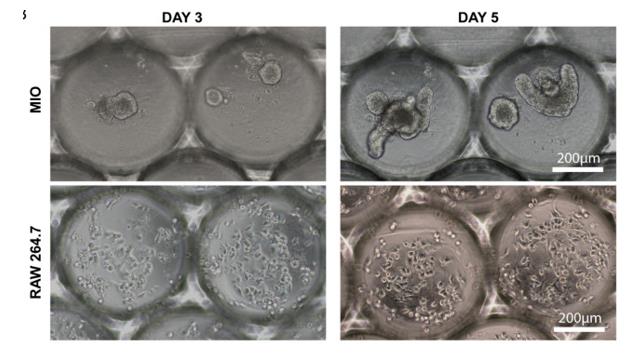
Fig1. Monocultures of organoids (MIO) and RAW 264.7 cells in microwells. Bright-field images demonstrating the growth of the organoids (top) and macrophages (bottom) over a period of 5 days.
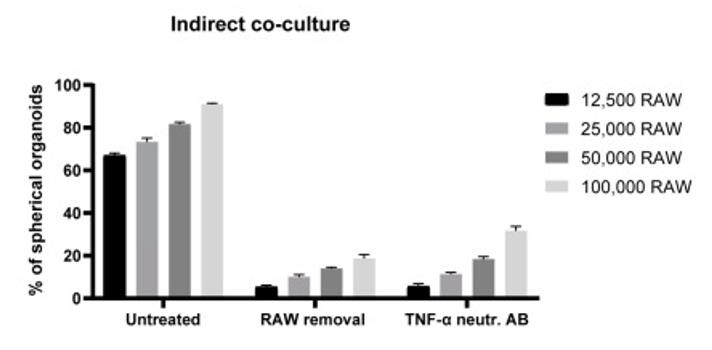
Fig2. TNF-α-induced effects on organoid morphology. Graph indicates the percentages of spherical organoids upon indirect co-culture with macrophages, indirect co-culture with subsequent removal of macrophages, and finally upon treatment with TNF-α neutralizing antibody on day 3 (500 ng/mL).
Case 4: Dijkstra KK, Cattaneo CM, Weeber F, et al. Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell. 2018 Sep 6;174(6):1586-1598.e12. doi: 10.1016/j.cell.2018.07.009. Epub 2018 Aug 9. PMID: 30100188; PMCID: PMC6558289.
The researchers establish and validate a platform to induce and analyze tumor-specific T cell responses to epithelial cancers in a personalized manner. They demonstrate that co-cultures of autologous tumor organoids and peripheral blood lymphocytes can be used to enrich tumor-reactive T cells from peripheral blood of patients with mismatch repair-deficient colorectal cancer and non-small-cell lung cancer. Furthermore, they demonstrate that these T cells can be used to assess the efficiency of killing of matched tumor organoids.
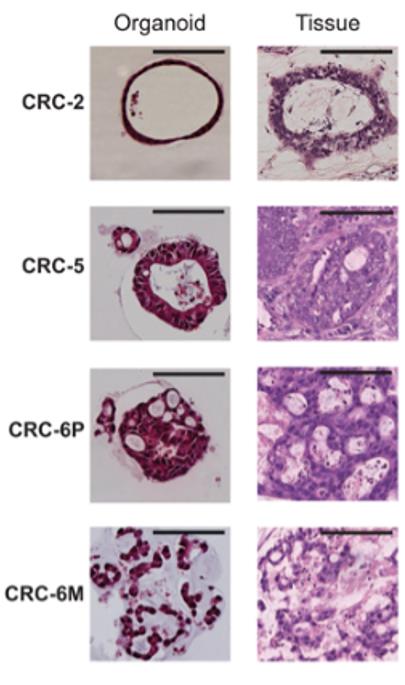
Fig1. Characterization of a panel of dMMR CRC organoids. (A) Haematoxylin and eosin (H&E) staining of tumor organoids and original tumor tissue. Organoids show morphology similar to the architecture of the original tumor. CRC-2 organoids demonstrates cystic tubules similar to mucin-filled glands of the primary tumor. CRC-5 organoids and primary tumor are composed of tubular glands with layered epithelium. Organoid CRC-6P is composed of complex cribriform glands, also seen in the primary tumor. Organoid CRC-6M consists of irregular trabecular structures and poorly formed glands, similar to the metastasis.
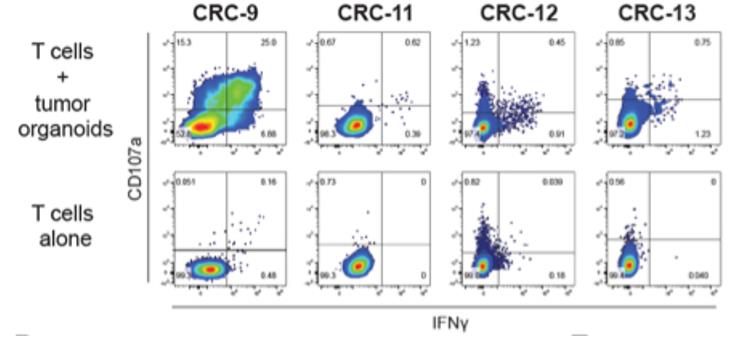
Fig2. Representative flow cytometry plots gated on CD8+ T cells tested for reactivity against autologous organoids after two weeks of co-culture with autologous tumor organoids.