Uncategorized Tuesday, 2025/05/13
Synthetic lethality
Synthetic lethality is not a new concept. It refers to the phenomenon where the simultaneous inhibition of two non-lethal genes leads to cell death. This holds extraordinary significance in cancer treatment, as this mechanism can be leveraged to develop drugs that specifically kill cancer cells without affecting normal cells. This has sparked a global rush among researchers to develop synthetic lethality targets.
Synthetic lethality Targets
Currently, among synthetic lethality targets, only PARP inhibitors have multiple products approved and marketed, including blockbusters like AstraZeneca's Lynparza, which has exceeded $2.5 billion in sales, highlighting the potential market value and development space for synthetic lethality targets.
After a period of quiet, synthetic lethality targets have once again seen licensing deals. On March 26, Bayer announced the introduction of a pipeline from Jiangsu's Pharmedic, obtaining global rights. This pipeline targets PRMT5, and Bayer has already recruited its first patient for a Phase I first-in-human dose-escalation clinical trial.
Our Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| PARP1-650H |
Recombinant Human PARP protein(Met1-Trp1014), His-tagged

|
Insect Cells | Human | His | Full L. 1-1014 a.a. | |
| PRMT5-1969H | Recombinant Human PRMT5 protein, GST-tagged | E.coli | Human | GST | 283-637 aa | |
| PRMT5-3528H | Recombinant Human PRMT5 protein, His-tagged | E.coli | Human | His | 283-637 aa | |
| PRMT5-3632H | Recombinant Human PRMT5 protein, GST-tagged | E.coli | Human | GST | 1-280 aa | |
| PRMT5-2321H | Recombinant Human PRMT5 protein, His&FLAG-tagged | HEK293 | Human | Flag&His | Ala2-Leu637 | |
| MTAP-386H | Recombinant Human MTAP Protein, His-tagged | E.coli | Human | His | Lys11~Pro256 | |
| Mtap-387H | Recombinant Mouse Mtap Protein, His-tagged | E.coli | Mouse | His | Ala6~Gln275 |
PRMT5 is not an obscure target, with many domestic biotech and biopharma companies investing in it, considering its potential in solid tumors, which should attract investors' attention.
Mechanism of PRMT5
PRMT stands for Protein Arginine Methyltransferase, which can methylate various proteins (including histones and non-histones). Nine members of the PRMT family have been discovered, named PRMT1-9.
In terms of the metabolic mechanism in the body, PRMT can methylate arginine in proteins to form methylarginine. As shown in the figure, PRMT can be categorized into three types based on the form of arginine generated: Type I, II, and III. PRMT5 belongs to Type II, catalyzing the formation of symmetrical dimethylarginine (SDMA) from its substrate. Its catalytic mechanism involves one of the substrates, S-adenosylmethionine (SAM), transferring a methyl group to arginine.
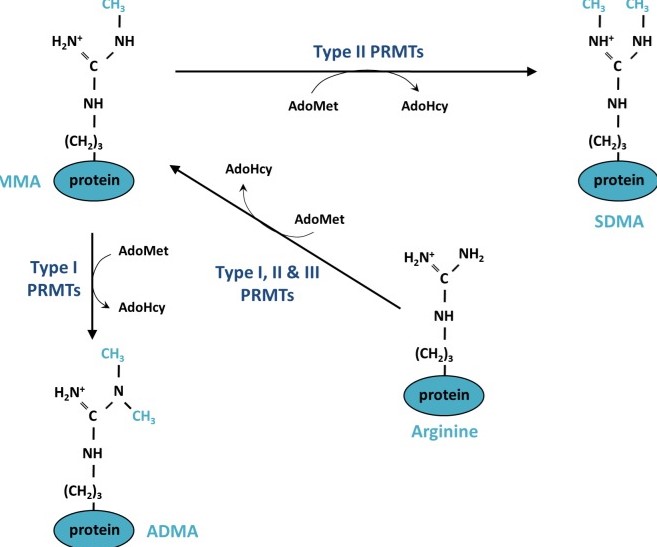
Fig1. Mechanism of protein methylation on arginine residues
In this process, PRMT5 plays a very complex role in tumorigenesis because it controls the expression of genes related to tumor promotion and suppression. It is noted that PRMT5 expression is upregulated in most types of cancer and, in most cases, this upregulation is associated with lower patient survival rates. In such a complex regulatory environment, it is conceivable that this methylation reaction could be positively linked to cancer progression and low survival rates, suggesting potential pathways to interrupt this reaction.
Researchers seem to have identified a pathway based on this foundation. As mentioned, one necessary substrate for this reaction is SAM, which can easily degrade into MTA (methylthioadenosine). MTA, in turn, is a competitive inhibitor of SAM for PRMT5. If MTA accumulates, the methylation reaction mediated by PRMT5 is halted. Thus, strategies to accumulate MTA could be developed. MTAP is an enzyme that can catalyze the degradation of MTA but is located near tumor suppressor genes, often deleted along with these genes in tumor patients, resulting in MTA accumulation, which negatively regulates PRMT5 methylation. Existing PRMT5 inhibitors exploit this by synergizing with MTA and targeting SAM, acting as competitive inhibitors to block the interaction between SAM and PRMT5.
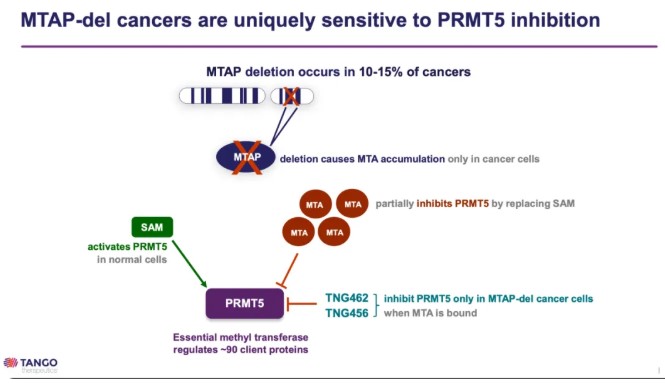
Fig2. PRMT5 inhibitor (Source: Tango's PPT)
Compounds Target PRMT5
Based on this, compounds have been developed following the mechanism described above, with representative molecules including AMG193 and GSK-3326595.
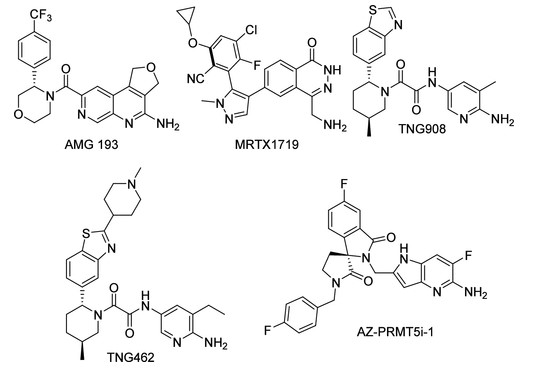
Fig3. Structures of the MTA-cooperative PRMT5 inhibitors currently in clinical trials AMG 193, MRTX1719, TNG908, and TNG462. Additionally, AZ-PRMT5i-1 is shown as it is a close analogue to AZD-3470, which is also in clinical trials.
However, the first-generation compounds have notable drawbacks: they cannot distinguish between normal cells and MTAP-deficient tumor cells, affecting normal cells and leading to significant side effects. In the first-in-human trial data for AMG193, 80 patients participated, and the occurrence of treatment-emergent adverse events (TEAEs) was 97.5%, with serious adverse events at 36.3%, and five patients discontinued due to adverse events.
Additionally, first-generation inhibitors are associated with bone marrow suppression, which can cause various hematological side effects such as anemia, leukopenia, and lymphopenia.
Currently, researchers are developing second-generation PRMT5 inhibitors, with Amgen's AM-9747 being a typical example. These compounds bind with the PRMT5-MTA complex, killing MTAP-deficient cancer cells while sparing normal cells. This selectivity allows them to target MTAP-deficient cancer cells more specifically, potentially reducing adverse event rates.
The selectivity of the "prototype" AM-9959 can be observed, which shows a ninefold increase in selectivity in the presence of MTA, where MTA forms a complex with PRMT5.
Efficacy
In terms of efficacy, second-generation inhibitors generally have not yet produced clinical data, so their effectiveness can only be speculated based on first-generation drugs.
First is the previously mentioned AMG193. In its Phase I clinical trial, patients received a median number of treatment lines of 2, with high malignancy cancer types like pancreatic cancer, non-small cell lung cancer, biliary tract cancer, and glioblastoma represented.
In terms of final efficacy, dose dependency was evident, with the 800mg cohort beginning to show a response, having 3 confirmed partial responses (PRs) and 1 unconfirmed PR, with an overall response rate (ORR) of 25%. The 1200mg cohort achieved an ORR of 29.4%. Though not stellar, these results are quite standard for a Phase I clinical trial. It's possible that Amgen considers AMG193 as an exploratory tool to gather clinical data from patients, with its primary focus on advancing the next-generation drug AM9747.
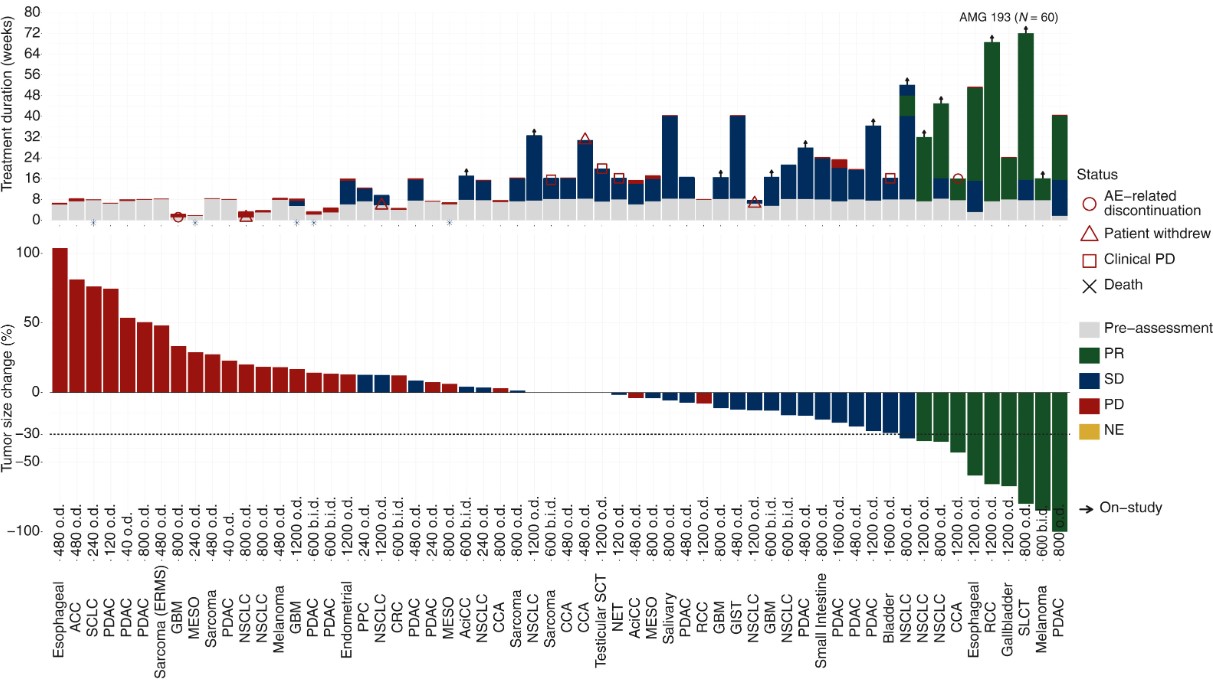
Fig4. Antitumor activity of AMG 193.
Another drug with rapid progression but unfortunate results is GSK's GSK-3326595. Despite being developed early, it has not been very successful. Phase I clinical results were available as early as 2019, and a search on the clinical trial website shows that all its clinical trials have either completed or terminated by 2022-2023, with no new trials initiated.
Regarding efficacy, only one patient with HR+ breast cancer achieved an ORR (out of 37 patients in this subgroup), with no ORR observed in other subgroups. In terms of safety, 95% of patients experienced treatment-related adverse events (TRAEs).
Additionally, the pipeline worth attention in this field includes TNG-462. According to its own description, it belongs to the second-generation inhibitors: capable of selectively killing cancer cells without affecting normal cells. This drug released data in November 2024.
As of October 20, 2024, 59 patients were enrolled, with 39 evaluable. Effective dosing ranged from 160-300mg/QD, and the current data show that in cholangiocarcinoma patients, three out of seven had an ORR of 43%. Although the sample size is not large, the company website compares it to Amgen's drug, but the comparison might not be persuasive with current small sample data. Nevertheless, this could signify the best start for PRMT5 inhibitors.
The advantage of this drug lies in truly realizing the selectivity of second-generation drugs, meaning very good safety. Reported adverse events include nausea, vomiting, diarrhea, and fatigue, occurring in less than 20% of patients, mainly grade 1. In addition, thrombocytopenia is a dose-limiting toxicity.
Moreover, Tango is trying to expand the potential uses for this drug, such as combination use with drugs like RAS inhibitors, osimertinib, and pembrolizumab, with plans to start patient recruitment in H1 2025.
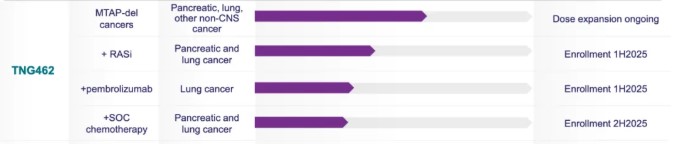
Fig5. Pipeline in Tango comany.
Conclusion
In conclusion, the article highlights the significance and evolving landscape of synthetic lethality in cancer treatment, with a particular focus on PRMT5 as a promising target. It underscores the therapeutic potential of PRMT5 inhibitors in selectively targeting cancer cells, especially those with MTAP deficiencies, while minimizing damage to normal cells. The development and clinical testing of both first-generation and second-generation inhibitors, such as AMG193 and TNG-462, demonstrate the ongoing efforts to enhance efficacy and safety. Although first-generation inhibitors faced challenges with side effects and selectivity, second-generation inhibitors are showing promise with improved specificity and reduced adverse events. The article suggests that continued research and development in this area, particularly through strategic collaborations and innovative approaches, could lead to significant advancements in cancer therapies targeting synthetic lethality.
Related Products & Services
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Immune Checkpoint Proteins
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Poulard C, Corbo L, Le Romancer M. Protein arginine methylation/demethylation and cancer. Oncotarget. 2016 Oct 11;7(41):67532-67550. doi: 10.18632/oncotarget.11376. PMID: 27556302; PMCID: PMC5341895.
- Sarvary, Ian, Vestergaard, Mikkel, et al. From DNA-Encoded Library Screening to AM-9747: An MTA-Cooperative PRMT5 Inhibitor with Potent Oral In Vivo Efficacy. J. Med. Chem. 2025, doi: 10.1021/acs.jmedchem.4c03101.
- Rodon J, Prenen H, Sacher A, Villalona-Calero M, Penel N, El Helali A, Rottey S, Yamamoto N, Ghiringhelli F, Goebeler ME, Doi T, Postel-Vinay S, Lin CC, Liu C, Chuang CH, Keyvanjah K, Eggert T, O'Neil BH. First-in-human study of AMG 193, an MTA-cooperative PRMT5 inhibitor, in patients with MTAP-deleted solid tumors: results from phase I dose exploration. Ann Oncol. 2024 Dec;35(12):1138-1147. doi: 10.1016/j.annonc.2024.08.2339. Epub 2024 Sep 16. PMID: 39293516.
