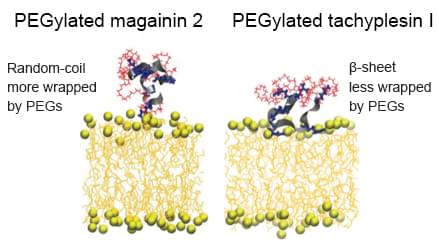
Uncategorized Friday, 2017/06/09
According to the report of Grand View Research, the demand for recombinant technology, pharmaceutical biotechnology, and DNA sequencing technology is increasing based on disease diagnosis and treatment requirement, and the global biotechnology market is expected to grow at a compound annual growth rate of 12.3%. By 2020, the market size will be $606.8 billion. Recombinant protein, a genetic engineering and cell engineering technology, produced from the creature body, has become the most important biotechnology production. Generalized recombinant protein drugs can be divided into the following three categories: recombinant protein, recombinant monoclonal antibody, and recombinant vaccine. Here we mainly analyze the narrow sense of recombinant protein drugs, that is, the recombinant protein. Recombinant protein drugs, also known as rDNA drugs, do not include recombinant vaccines, monoclonal antibody drugs, recombinant proteins for detection, biochemical extraction of natural proteins, and generic drugs. Recombinant protein drugs have accounted for about 10% of the global prescription drug market. The market development was rapid, especially in the 21st century, which could be called the golden age. The sale reached 4.7 billion in1989, 28.5 billion in 2001, 34.7 billion in 2004, about 41 billion in 2005 and more than 100 billion in 2011, which was 20 times compared to 1989. The annual compound growth rate was 15%, which was extremely faster than the world GDP growth rate at the same period. The United States and Europe account for 81% of the global market. The biggest recombinant protein drug research firms (Amgen, Biogen IDEC, Johnson & Johnson, Eli Lily, Novo Nordisk, and Roche) are all from the United States or Europe, occupies 75% market share. From the number and speed of new drugs listed, the United States ranks first, which has a clear relationship with the freer drug price environment and high acceptance of new drugs for the doctors in the US. Europe has developed rapidly in recent years, taking the lead in approving the listing of recombinant human antithrombin (GTC biotherapeutic company), which is produced by transgenic animals (sheep). 1. Advantages for market and research The recombinant protein is effective and plays an irreplaceable role in the treatment of certain diseases. Such as hemophilia, except blood coagulation factor, which has an extremely limited source, it can only rely on the recombinant coagulation factor. Most recombinant protein drugs’ applies to diseases due to a single gene or single protein loss or function loss. Recombinant protein drugs are subject to higher pricing due to higher technical barriers and financial barriers. The biological function of the recombinant protein is highly correlated with the folding and modification of the peptide, which determines that its research and development is much more difficult than small molecule chemical drugs. Recombinant protein production depends on a variety of biosynthesis systems, including choosing from bio-engineering E. coli, yeast, mammalian cells, etc. The determination of production system selection, vector construction, parameter optimization, separation and purification, activity testing and other steps depends on long-term theoretical guidance, experience, and practical results. The recombinant protein is more difficult to replicate than small molecule chemicals. Unlike small molecule chemicals, even if the same gene is expressed in the same cell and uses a similar processing method, the recombinant drug generic drug is difficult to guarantee exactly the same as the original.Besides, recombinant protein drugs are targeted at major diseases areas, such as cancer, viral diseases, cardiovascular diseases and endocrine diseases. The recombinant protein market has a large extension space and relatively few approval drugs. Additionally, most of the drugs have no substituted product in their respective therapeutic areas. In short, the vast majority of recombinant protein drugs are human proteins or their mutants to compensate for some of the functional proteins in vivo defects or increase the body's protein function. Thus, recombinant protein drug has significantly greater safety than small molecules, and lead to a higher approval rate. At the same time, its clinical trial period is shorter than the small molecule drugs, patent protection is relatively extended, which gives the pharmaceutical company longer exclusive sales time. These characteristics have become an important motion for the development of recombinant protein drugs.
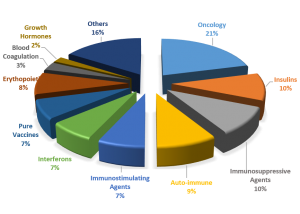 Fig1. Market share of the Leading Therapy Classes, 2015 (estimated)
Fig1. Market share of the Leading Therapy Classes, 2015 (estimated)
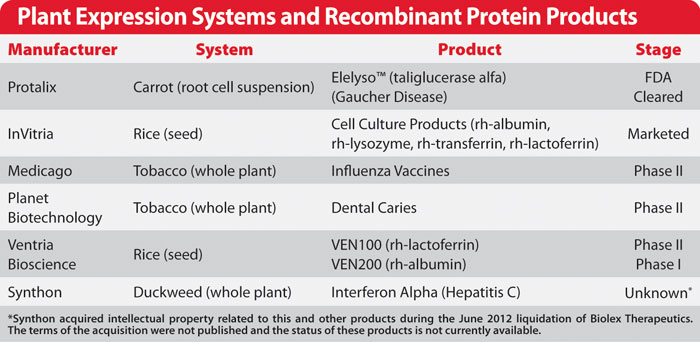 Fig2. Plant expression systems and recombinant protein products
Fig2. Plant expression systems and recombinant protein products
 3) Administration approaches change
The majority of recombinant protein drugs are injectable or intravenous. But some diseases, such as diabetes, renal failure anemia, etc. require long-term use of drugs, injection or intravenous approach is very inconvenient. So there is a lot of experimentation going on improving administration approaches, for example, pharmaceutical spray. In this process, the biggest technical difficulty is the dose accuracy and drug stability.
4. Problems and challenges for recombinant protein drugs
1) Other therapeutic drugs or methods challenge the market share of recombinant drugs
Many of the indications of recombinant drugs are due to the lack of a single gene or a simple cause induced protein lacking or function losing, such as hemophilia and type I diabetes. These symptoms are suitable for gene therapy too. Imagine, if the body can be regulated to express the recombinant drug-gene, how about the future of recombinant drug? Will gene therapy affect the recombinant drug market in the short term? The fact is that since 1989, about 1140 gene therapy products have entered clinical studies, with only a few entering clinical phase III studies, but none of them are listed in the US and Europe, and many have terminated due to accidental death in clinical studies. Inducing stem cell to form islet-like cell has not progressed as expected, there will be longer distant to achieve clinical therapy than gene therapy. The mechanism of antibody drugs or aptamer is specific target binding and inhibition of the conjugate, but the mechanism of majority recombinant drugs are complementary. So either the antibody drug or aptamer only challenges the market of recombinant drugs with inhibition mechanisms, such as Enbrel and β-interferon.
2) Effect of recombinant drug generic drugs on recombinant drug market
The current rule of the drug market is: after the new drug patent protection period, there will be generic drugs listed, whose price is about 15% of new drugs. There will be a great impact on drug profits.
3) Clinical safety risk
Like other drugs, recombinant drugs also have the risk of causing side effects. First, the function of the recombinant drug is not unique. Secondly, its effect degree is difficult to control precisely, which may lead to serious side effects. Clinical safety risk directly influences the speed of approval of new drugs, which also affects the ma
3) Administration approaches change
The majority of recombinant protein drugs are injectable or intravenous. But some diseases, such as diabetes, renal failure anemia, etc. require long-term use of drugs, injection or intravenous approach is very inconvenient. So there is a lot of experimentation going on improving administration approaches, for example, pharmaceutical spray. In this process, the biggest technical difficulty is the dose accuracy and drug stability.
4. Problems and challenges for recombinant protein drugs
1) Other therapeutic drugs or methods challenge the market share of recombinant drugs
Many of the indications of recombinant drugs are due to the lack of a single gene or a simple cause induced protein lacking or function losing, such as hemophilia and type I diabetes. These symptoms are suitable for gene therapy too. Imagine, if the body can be regulated to express the recombinant drug-gene, how about the future of recombinant drug? Will gene therapy affect the recombinant drug market in the short term? The fact is that since 1989, about 1140 gene therapy products have entered clinical studies, with only a few entering clinical phase III studies, but none of them are listed in the US and Europe, and many have terminated due to accidental death in clinical studies. Inducing stem cell to form islet-like cell has not progressed as expected, there will be longer distant to achieve clinical therapy than gene therapy. The mechanism of antibody drugs or aptamer is specific target binding and inhibition of the conjugate, but the mechanism of majority recombinant drugs are complementary. So either the antibody drug or aptamer only challenges the market of recombinant drugs with inhibition mechanisms, such as Enbrel and β-interferon.
2) Effect of recombinant drug generic drugs on recombinant drug market
The current rule of the drug market is: after the new drug patent protection period, there will be generic drugs listed, whose price is about 15% of new drugs. There will be a great impact on drug profits.
3) Clinical safety risk
Like other drugs, recombinant drugs also have the risk of causing side effects. First, the function of the recombinant drug is not unique. Secondly, its effect degree is difficult to control precisely, which may lead to serious side effects. Clinical safety risk directly influences the speed of approval of new drugs, which also affects the ma