Adhesion Molecules in Angiogenesis
Cell adhesion molecules (CAMs) are a subset of cell adhesion proteins located on the cell surface that are involved in binding to other cells or extracellular matrices (ECMs), called cell adhesion. In essence, cell adhesion molecules can help cells adhere to each other and adhere to the surrounding environment. Cell adhesion is a key component in maintaining tissue structure and function. In fully developed animals, these molecules play an integral role in the production of forces and movements, thus ensuring that organs can perform their functions. In addition to its use as a "molecule gel," cell adhesion is also important in mechanisms that affect cell growth, contact inhibition, and apoptosis. Often, abnormal expression of CAMs can lead to a variety of diseases, from frostbite to cancer.
Structure
CAM is typically a single-pass transmembrane receptor (Figure 1) composed of three conserved domains: an intracellular domain that interacts with the cytoskeleton, a transmembrane domain, and an extracellular domain. These proteins can interact in several different ways. The first method is by homologous binding in which the CAM binds to the same CAM. They also have heterologous binding capabilities, which means that the CAM on one cell will bind to a different CAM on another cell. The final type of binding occurs between the cell and the substrate, where two mutually different ligands of different CAMs are bound.
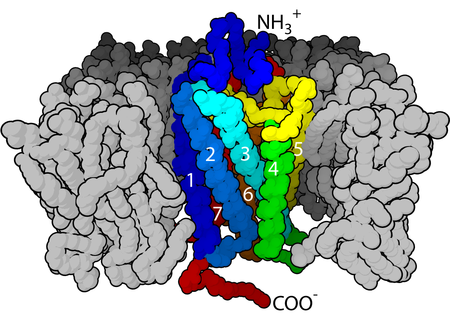 Figure 1. The seven-transmembrane α-helix structure of a G-protein-coupled receptor.
Figure 1. The seven-transmembrane α-helix structure of a G-protein-coupled receptor.
Families of CAMs
CAM has four major superfamilies or groups: the cell adhesion molecule (IgCAM) of the immunoglobulin superfamily, the cadherin, the integrin, and the C-type lectin-like domain protein superfamily (CTLD). Proteoglycans are also considered to be a class of CAMs. One classification system involves the distinction between calcium-independent CAM and calcium-independent CAM. Integrins and Ig superfamily CAM are not dependent on Ca2+, whereas cadherins and selectins are dependent on Ca2+. In addition, integrins are involved in cell-matrix interactions, while other CAM families are involved in cell-cell interactions.
Calcium-independent
Integrins
Integrins (Figure 2) are one of the major receptors in ECM and mediate the interaction of cells with ECM with collagen, fibrinogen, fibronectin and vitronectin. Integrins provide an important link between the extracellular environment and intracellular signaling pathways that can play a role in cellular behavior, such as apoptosis, differentiation, survival, and transcription. Integrins are heterodimers because they consist of alpha and beta subunits. There are currently 18 alpha subunits and 8 beta subunits which combine to form 24 different integrin combinations. Within each of the alpha and beta subunits, there is a large extracellular domain, a transmembrane domain and a short cytoplasmic domain. The extracellular domain is where the ligand binds by using divalent cations. Generally, Mn2+ increases affinity, Mg2+ promotes cell adhesion, and Ca2+ reduces cell adhesion. Integrin regulates its activity in the body by changing its conformation, most are present in a low affinity state at rest and can be altered to high affinity by an external agonist, causing a conformational change in the integrin, thereby increasing their affinity. An example is the aggregation of platelets. An agonist such as thrombin or collagen triggers integrin into its high affinity state, which leads to increased fibrinogen binding leading to platelet aggregation.
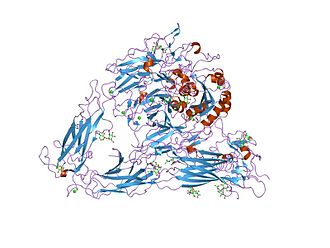 Figure 2. Structure of the extracellular segment of integrin alpha Vbeta3
Figure 2. Structure of the extracellular segment of integrin alpha Vbeta3
IgSF CAMs
The immunoglobulin superfamily CAM (IgSF CAM) is considered to be the most diverse superfamily of CAM. This family is characterized in that its extracellular domain comprises an Ig-like domain. Following the Ig domain is a type III fibronectin domain repeat, and IgSF is anchored to the membrane by the GPI moiety. This family is involved in homotypic or heterotypic binding and has the ability to bind to integrins or different IgSF CAMs.
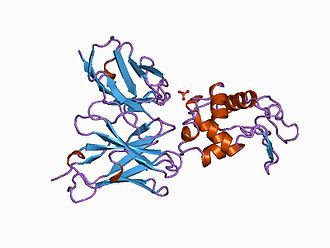 Figure 3. Antibody in complex with hen egg white lysozyme
Figure 3. Antibody in complex with hen egg white lysozyme
Calcium-dependent
Cadherins
Cadherin is a homologous Ca2+-dependent glycoprotein. The classical cadherin is concentrated at the junction of the intermediate cells, and the intermediate junction is linked to the actin filament network by a specific connexin called catenin. Cadherin is noteworthy in embryonic development. For example, the formation of cadherin for mesoderm, endoderm and ectoderm is critical in the stomach. Cadherin also makes an important contribution to the development of the nervous system. The unique temporal and spatial localization of cadherin suggests that these molecules play a major role in synaptic stabilization. Each cadherin exhibits a unique pattern of tissue distribution and is carefully controlled by calcium. Diverse families of cadherin include epithelial (E-cadherin), placenta (P-Cadherin), nerve (N-Cadherin), retina (R-Cadherin), brain (B-Calcium adhesion) Protein and T-cadherin) and muscle (M-calmodulin). Many cell types express a combination of cadherin types. The extracellular domain has a major repeat sequence called the extracellular cadherin domain (ECD). Sequences involved in Ca2+ binding between ECDs are essential for cell adhesion. The cytoplasmic domain has a specific region of catenin binding.
Selectins
The selectin (Figure 4) is a heterologous CAM family that is dependent on fucosylated carbohydrate (eg, mucin) binding. The three family members are E-selectin (endothelial cells), L-selectin (white blood cells) and P-selectin (platelets). The most characteristic of the three selectins is P-selectin glycoprotein ligand 1 (PSGL-1), a mucin-type glycoprotein expressed on all white blood cells. Selectins have been involved in a variety of roles, but they are especially important in the immune system by helping white blood cells to homing and transport.
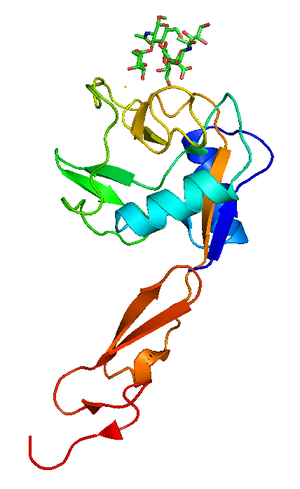 Figure 4. Crystallographic structure of P-selectin lectin bound to sugar, shown in sticks.
Figure 4. Crystallographic structure of P-selectin lectin bound to sugar, shown in sticks.
Biological function of CAMs
The diversity of CAMs leads to the multiple functions of these proteins in biological environments. A type of CAMS that is particularly important in the homing of lymphocytes is called Addressins. Lymphocyte homing is a key process occurring in a strong immune system. It controls the process of circulating lymphocytes adhering to specific areas and organs of the body. This process is highly regulated by cell adhesion molecules, especially the addressing protein. This antigen is known for its role in tissue-specific adhesion of lymphocytes to high endothelial venules. Through these interactions, they play a crucial role in coordinating circulating lymphocytes. The function of CAM in cancer metastasis, inflammation and thrombosis makes it a viable therapeutic target currently under consideration. For example, they prevent the ability of metastatic cancer cells to spread and transfer to secondary sites. This has been successfully demonstrated in metastatic melanoma that is pronounced in the lungs. In mice, when an antibody against CAM in the pulmonary endothelium is used as a therapeutic agent, the number of transfer sites is significantly reduced.
References:
1. Korthuis RJ.; et al. Role of neutrophil-endothelial cell adhesion in inflammatory disorders. J Crit Care. 1994, 9 (1): 47-71.
2. Chothia C.; et al. The molecular structure of cell adhesion molecules. Annu. Rev. Biochem. 1997,66: 823-62.


