Reperfusion Injury Therapeutic Targets
Available Resources for Study of Reperfusion Injury Therapeutic Targets
Creative BioMart is committed to being at the forefront of reperfusion injury research. Our priority is to ensure that researchers have access to the latest tools and information to drive progress in this important field of study.
- We offer a wide range of products, including recombinant proteins and other essential materials that play a crucial role in uncovering the mechanisms of reperfusion injury. We understand that each researcher has unique requirements, which is why we provide a diverse selection of products tailored to their specific needs.
- Our team of experienced experts in reperfusion injury research are incredibly knowledgeable and dedicated to their work. They are committed to providing customized and individualized solutions to meet the specific requirements of every researcher.
- In addition, we offer comprehensive support resources that cover involved pathways, protein functions, interacting proteins, and other valuable information. Our goal is to empower researchers to gain a comprehensive understanding of reperfusion injury and amplify the impact of their research.
Our Featured Products
- Recombinant Human STAT1 protein (Met1-Val712), His&GST-tagged
- Recombinant Human STAT1 protein, GST-tagged
- Recombinant Human STAT1, His-tagged
- Recombinant Human PRKCD, His-tagged
- Active Recombinant Human CCL2 Protein, His-tagged
- Recombinant Human MAPK14 protein (Met1-Ser360), His-tagged
- Recombinant Human Albumin, Animal Free, Antibiotics Free
- Recombinant Human ALB, His-tagged
Whether you are studying reperfusion injury pathology, drug discovery, or treatment development, Creative BioMart is here to support your research. We are committed to helping you achieve your scientific goals and make meaningful contributions to the field of reperfusion injury research. Contact us today to learn more about our products and resources.
About Reperfusion Injury
Reperfusion injury refers to the damage that occurs when blood supply returns to tissues or organs after a period of ischemia (lack of oxygen and nutrient supply). While reperfusion is essential for restoring blood flow and preventing tissue death, it can paradoxically lead to additional damage due to the generation of reactive oxygen species (ROS), inflammation, and cell death.
Causes of Reperfusion Injury
- Ischemia and subsequent reperfusion can occur in various clinical situations, such as during heart attacks, strokes, organ transplantation, or surgeries that involve interruption of blood flow.
- The extent of reperfusion injury depends on the duration and severity of the preceding ischemic period. Prolonged ischemia leads to more severe injury upon reperfusion.
Developmental Mechanisms of Reperfusion Injury
- Reactive Oxygen Species (ROS): The sudden reintroduction of oxygen during reperfusion leads to the production of ROS, which are highly reactive molecules that can damage lipids, proteins, and DNA. ROS-induced oxidative stress contributes to cell and tissue damage.
- Calcium Overload: Ischemic conditions disrupt calcium homeostasis in cells. When blood flow is restored, an excessive influx of calcium ions occurs, which can activate various enzymes leading to cell death and tissue damage.
- Inflammation: Reperfusion triggers an inflammatory response, mediated by the activation of immune cells and the release of pro-inflammatory molecules. This inflammatory cascade further contributes to tissue damage.
- Endothelial Dysfunction: Ischemia-reperfusion injury affects the endothelial lining of blood vessels, leading to impaired vascular function. This dysfunction can result in decreased blood flow, increased permeability, and vasoconstriction, further exacerbating tissue damage.
- Mitochondrial Dysfunction: Ischemia and reperfusion disrupt mitochondrial function, reducing cellular energy production and causing the release of pro-apoptotic factors. Mitochondrial dysfunction plays a significant role in reperfusion injury.
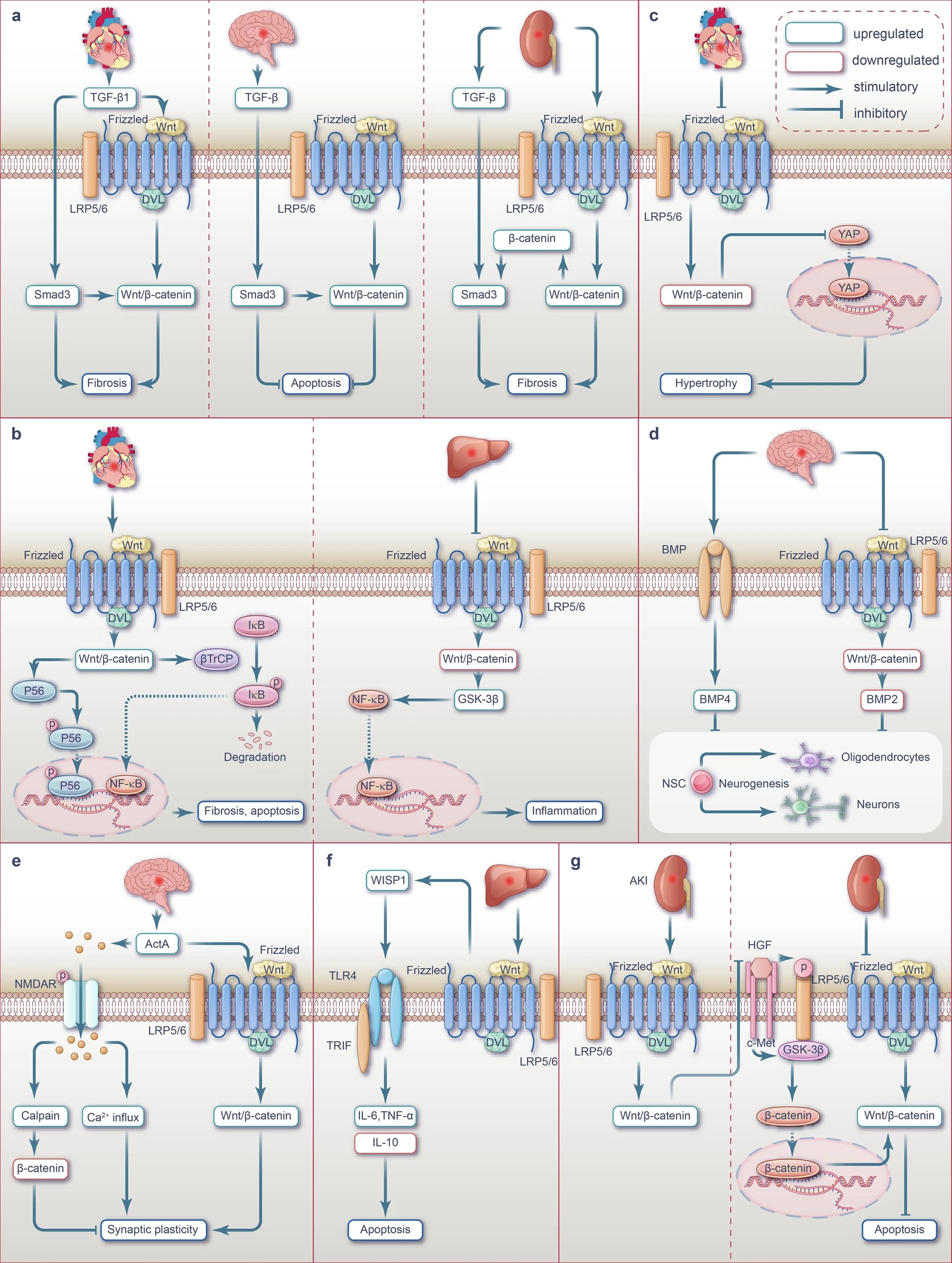 Fig.1 Interplay between Wnt, TGF-β, NF-κB, Hippo-YAP, BMP, NMDAR-Ca2+-ActA, TLR4/TRIF and HGF/c-Met signaling pathways during ischemia or I/R injury. (Mikami Y, et al., 2023)
Fig.1 Interplay between Wnt, TGF-β, NF-κB, Hippo-YAP, BMP, NMDAR-Ca2+-ActA, TLR4/TRIF and HGF/c-Met signaling pathways during ischemia or I/R injury. (Mikami Y, et al., 2023)
a Crosstalk between Wnt and TGF-β signaling pathway during ischemia injury.
b Crosstalk between Wnt and NF-κB signaling pathway during ischemia or I/R injury.
c Crosstalk between Wnt and Hippo-YAP signaling pathway during I/R injury.
d Crosstalk between Wnt and BMP signaling pathway during ischemia injury.
e Crosstalk between Wnt and NMDAR-Ca2+-ActA signaling pathway during I/R injury.
f Crosstalk between Wnt and TLR4/TRIF signaling pathway during I/R injury.
g Crosstalk between Wnt and HGF/c-Met signaling pathway during I/R injury.
The Treatment Targets of Reperfusion Injury
Therapeutic targets for reperfusion injury are actively being investigated to develop interventions that can mitigate tissue damage and improve outcomes. Several key targets have been identified, including cytokines, receptors, and signaling pathways. Here are some of the therapeutic targets and their related molecular mechanisms and pathogenic pathway analyses in the context of reperfusion injury:
Reactive Oxygen Species (ROS)
- ROS plays a central role in reperfusion injury by causing oxidative stress and cellular damage.
- Antioxidant therapies, such as superoxide dismutase (SOD) mimetics and catalase, aim to scavenge ROS and reduce oxidative stress.
Inflammatory Mediators
- Inflammatory response contributes to reperfusion injury. Targeting specific cytokines and chemokines involved in the inflammatory cascade is being explored.
- Interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) are potential targets, and their inhibition may attenuate the inflammatory response and reduce tissue damage.
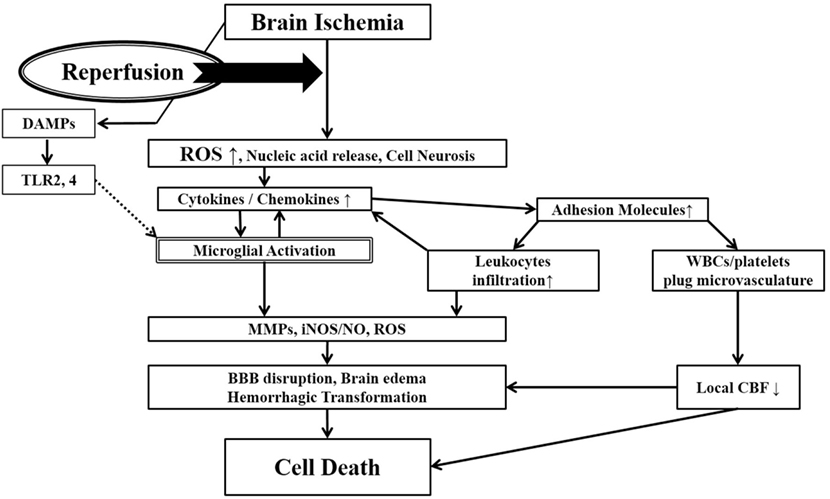 Fig.2 Ischemia-induced inflammation in association with reperfusion injury. (Mizuma A, et al., 2017)
Fig.2 Ischemia-induced inflammation in association with reperfusion injury. (Mizuma A, et al., 2017)
Once brain ischemia occurs, oxygen and glucose supplies are reduced. If ischemia occurs for more than a certain time period (likely a few hours, but the precise duration is not well established) and blood flow is restored (reperfusion), worsened injury can paradoxically occur to the brain. This is often referred to as reperfusion injury. A major component of reperfusion injury involves subsequent inflammatory reactions induced through various mechanisms. Reperfusion leads to the introduction of ROS from oxygenated blood and can stimulate an immune response in the ischemic brain. Necrotic, ischemia-injured cells lyse and release their contents into the extracelluar space which can act as ligands for various immune receptors. Among these include nucleic acids which are one of many described damage-associated molecular pattern (DAMPs, see text for details). DAMPs can then bind TLRs and stimulate several inflammatory responses (microglial activation, overexpression of proinflammatory cytokines, chemokines) which lead to worsened brain injury. Inflammatory signaling also causes immune cells to generate more effector molecules such as ROS and iNOS/NO. In the periphery, cytokines and adhesion molecules can attract circulating immune cells to the ischemic brain where they infiltrate the damaged tissue and further amplify ischemic injury. Some circulating immune cells and platelets can also plug the microvasculature of the ischemic brain and cause secondary reductions in local CBF. In addition to brain cells, these inflammatory reactions can also cause damage to brain endothelia causing BBB disruption, edema and hemorrhagic transformation. Thus, the restoration of CBF can cause more extensive brain tissue damage. This vicious cycle is often called reperfusion injury. ROS, reactive oxygen species; DAMPs, damage-associated molecular patterns; TLR, toll-like receptor; MMPs, matrix metalloproteinases; iNOS, inducible nitric oxide synthase; NO, nitric oxide; BBB, blood–brain barrier; CBF, cerebral blood flow.
Toll-like Receptors (TLRs)
- TLRs play a crucial role in initiating the inflammatory response following reperfusion injury.
- Inhibiting TLR signaling, such as blocking TLR4, can limit inflammation and tissue damage.
Nitric Oxide (NO) Pathway
- NO is a signaling molecule involved in regulating blood flow and vascular function.
- Enhancing NO production or using NO donors may promote vasodilation and improve blood flow during reperfusion.
Mitochondrial Function
- Mitochondrial dysfunction contributes to reperfusion injury. Targeting mitochondrial pathways is being investigated.
- Agents that stabilize mitochondrial membranes, modulate mitochondrial permeability transition pore (mPTP) opening, or enhance mitochondrial bioenergetics are potential therapeutic strategies.
Cell Death Pathways
- Apoptosis and necrosis are involved in reperfusion injury-induced cell death.
- Inhibitors of apoptotic pathways, such as caspase inhibitors, or modulators of necrosis, such as necrostatins, are being explored to limit cell death and tissue damage.
Adenosine Receptors
- Adenosine, an endogenous nucleoside, exhibits protective effects during reperfusion injury.
- Activation of adenosine receptors can reduce inflammation, improve vascular function, and protect against tissue damage.
Calcium Handling
- Calcium overload during reperfusion contributes to cellular damage.
- Targeting calcium channels or modulating calcium-handling proteins, such as the sodium-calcium exchanger, can help restore calcium homeostasis and mitigate reperfusion injury.
Endothelial Function
- Endothelial dysfunction is a hallmark of reperfusion injury. Targeting endothelial pathways involved in inflammation and vascular permeability is being explored.
- Strategies include enhancing endothelial nitric oxide synthase (eNOS) activity, inhibiting adhesion molecules, and modulating endothelial barrier function.
These are some of the therapeutic targets and related molecular mechanisms being investigated in the context of reperfusion injury. It's important to note that the specific targets and approaches may vary depending on the organ or tissue affected by reperfusion injury. Further research is needed to develop effective therapies that can mitigate tissue damage and improve outcomes in patients experiencing reperfusion injury.
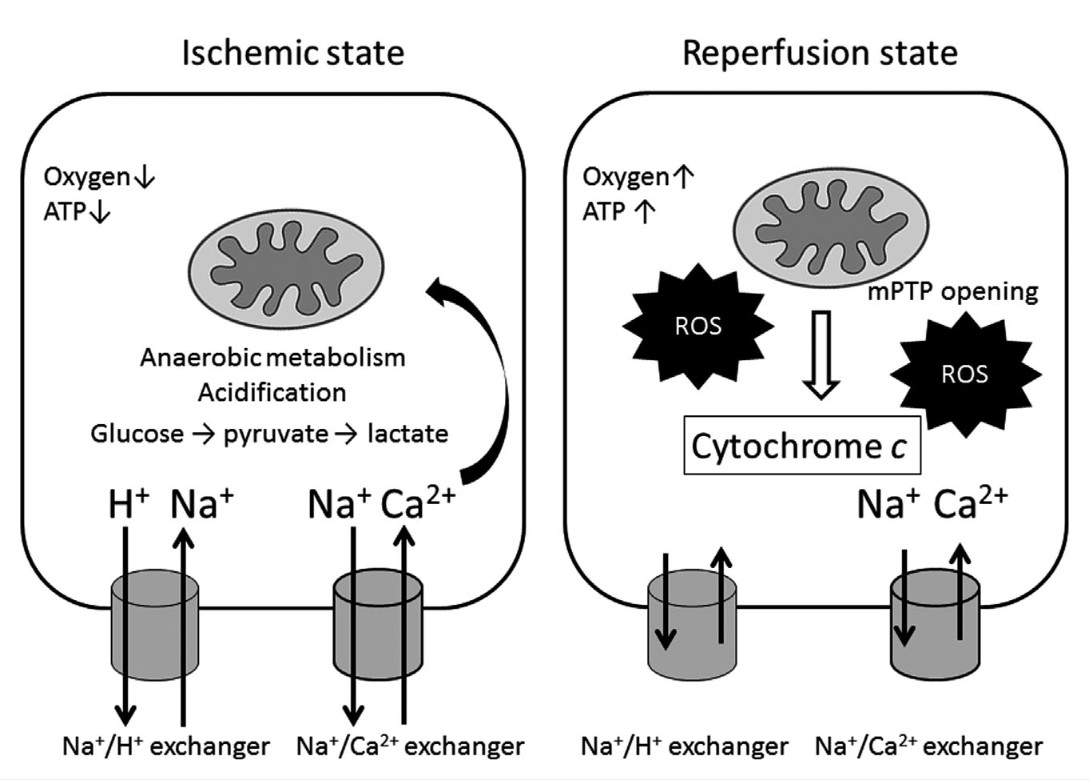 Fig.3 Schematic images of sequential changes in cytosolic and mitochondrial function during ischemia and reperfusion injury. (Naito H, et al., 2020)
Fig.3 Schematic images of sequential changes in cytosolic and mitochondrial function during ischemia and reperfusion injury. (Naito H, et al., 2020)
During hypoxia, reduced O2 promotes anaerobic glycolysis that generates increased cytosolic lactate leading to acidification. Increased H+ activates Na+-H+ exchanger leading to increased cytosolic Na+, which activates Na+-Ca2+ exchanger, causing an increase in cytosolic Ca2+. Cytosolic Ca2+ overload in turn increases mitochondrial matrix Ca2+. Impaired electron transport leads to increased generation of reactive oxygen species (ROS). Impaired respiration and substrate utilization lead to decreased generation of mitochondrial adenosine triphosphate (ATP). During the reperfusion state, an increased mitochondrial permeability transition pore (mPTP) opening elevates ROS generation and disrupts intracellular distribution of Ca2+, Na+, and pH, resulting in subsequent irreversible cell death. ROS further increase to produce even greater mitochondria damage that induces mPTP opening and release of cytochrome c that in turn triggers apoptosis.
References:
- Mikami Y, Kanai T. Network Approaches to Uncover Pathogenesis and Therapeutic Targets of Inflammatory Bowel Diseases. Keio J Med. 2023;72(2):29-43. doi:10.2302/kjm.2022-0015-IR
- Agrawal M, Colombel JF. Treat-to-Target in Inflammatory Bowel Diseases, What Is the Target and How Do We Treat? Gastrointest Endosc Clin N Am. 2019;29(3):421-436. doi:10.1016/j.giec.2019.02.004
- Mizuma A, Yenari M A. Anti-inflammatory targets for the treatment of reperfusion injury in stroke[J]. Frontiers in neurology, 2017, 8: 467.
- Naito H, Nojima T, Fujisaki N, et al. Therapeutic strategies for ischemia reperfusion injury in emergency medicine[J]. Acute medicine & surgery, 2020, 7(1): e501.


