Ubiquitin
About Ubiquitin
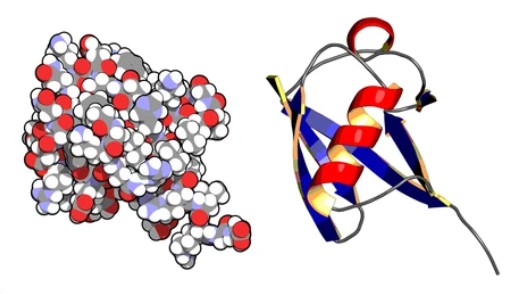
Ubiquitin is a small protein present in most eukaryotic cells, consisting of 76 amino acids, with a molecular weight of about 8,500 daltons, and is highly conserved in eukaryotes. Its main function is to tag proteins that need to be broken down so that they can be hydrolyzed. Ubiquitin can also label transmembrane proteins, such as receptors, to remove them from the cell membrane. Proteins that need to be degraded by the proteasome are first labeled by attaching ubiquitin, which is a covalent linkage between a lysine on the protein and ubiquitin. This process is a three-enzyme cascade reaction that requires the participation of three enzymes (E1, E2, and E3).
The ubiquitin (Ub) system plays a key role in protein homeostasis by regulating the turnover of proteins important in numerous regulatory pathways (DNA damage and repair, cell cycle progression, apoptosis, receptor-mediated endocytosis, and signaling, among others). Alterations in this pathway usually lead to pathological conditions in the host.
The Ubiquitination Signaling Pathway
The entire process by which the ubiquitination reaction occurs is known as the ubiquitination signaling pathway (Ub pathway.) or ubiquitin-proteasome pathway (UPP). The Ub pathway involves an unusual combination of many specific enzymatic proteins that target virtually all of the short-lived and abnormal proteins for proteasomal degradation. The half-life of each protein is precisely regulated, and degradation is extremely selective and controlled at the level of ubiquitination. The role of Ub as a signal for protein hydrolysis and the mechanism by which the high degree of organization required for the formation of an isopeptide bond between Ub and the substrate has been well characterized. The entire enzymatic cascade reaction is as follows:
- In the first step of the reaction, the ubiquitin-activating enzyme (also known as E1) hydrolyzes ATP and adenylates a ubiquitin molecule.
- Next, ubiquitin is transferred to a cysteine residue in the active center of E1 and is accompanied by the adenylation of a second ubiquitin molecule. The adenylated ubiquitin molecule is then transferred to a cysteine residue of ubiquitin cross-linking enzyme (E2).
- Finally, a member of the highly conserved ubiquitin ligase (E3) family recognizes a specific target protein (which varies depending on the substrate protein) that needs to be ubiquitinated and catalyzes the transfer of the ubiquitin molecule off of E2 to the target protein. The target protein must be labeled with at least four ubiquitin monomer molecules (in the form of polyubiquitin chains) before it can be recognized by the proteasome. Thus, it is E3 that makes this system substrate-specific. (The number of E1, E2, and E3 proteins is dependent on the organism and cell type, and the large number of different E3 proteins present in the human body suggests that the ubiquitin-proteasome system can act on a huge number of target proteins.)
- Ultimately, labeled proteins are broken down by proteases into smaller peptides, amino acids, and reusable ubiquitin.
Functions of Ubiquitin
Ubiquitin is primarily known for its role in the degradation of proteins through the ubiquitin-proteasome pathway (UPP). This pathway involves the covalent attachment of ubiquitin to target degraded proteins, marking them for recognition by the proteasome. The UPP plays a key role in maintaining protein homeostasis, regulating cell cycle progression, and responding to cellular stress. Dysregulation of this pathway has been linked to a variety of diseases, including cancer, neurodegenerative diseases, and immune-related disorders.
At the same time, ubiquitin is involved in several other important cellular processes, such as DNA repair, cell signaling, and chromatin remodeling. For example, ubiquitination can act as a post-translational modification that affects the function, stability, or localization of target proteins. It participates in the DNA repair pathway by promoting the recruitment of damaged DNA repair factors.
In addition, ubiquitin-dependent signaling regulates various aspects of cellular physiology, including immune responses, cell division, and development.
Available Resources of Ubiquitin
Creative BioMart is a leading biotechnology company offering high-quality products related to ubiquitin research for studying ubiquitin-related processes such as ubiquitination, deubiquitination, and protein degradation. We offer products related to NEDD4L and UBB. Product types include recombinant proteins, antibodies, protein pre-coupled magnetic beads, and cell & tissue lysates. In addition to our products and customized services, we offer ubiquitin-related signaling pathways, protein functions, interacting proteins, research areas, and other resources to support the study of various biological processes and pathways.
Ubiquitin is a fundamental regulatory protein involved in a wide range of cellular processes with broad implications in biology and medicine. Understanding the functional applications and mechanisms associated with ubiquitin is critical to unraveling its role in health and disease. Creative BioMart is committed to ubiquitin research and offers a comprehensive range of products, customized services, and other resources. Our commitment to quality and customer satisfaction makes us a trusted partner in the scientific community for the advancement of ubiquitin research. Please note that the product types and resources of each ubiquitin are different, please contact us with any questions or requests.


