Principle and Protocol of Protein Concentration Measurement by UV
Tyrosine, tryptophan and phenylalanine (containing the conjugated double bond of benzene ring structure) in proteins make most proteins have the maximum absorption peak at the wavelength of 280 nm. Therefore, using this specific absorption, a spectrophotometer can be used to directly test protein samples at the wavelength of 280 nm, and the protein content can be calculated using the standard curve or according to Warburg Christian formula. This direct quantitative method for protein is suitable for the determination of relatively pure and single protein. If there are interfering substances such as nucleic acid, the results will be inaccurate. At the same time, the results will be unstable due to the heterogeneity of the sample. However, the UV absorption method is fast, simple, and the sample can be recovered, so it is relatively widely used in a certain range.
Tyrosine in protein molecules (maximum absorption wavelength λmax is 275 nm), tryptophan (λmax is 280 nm) and phenylalanine (λmax is 257 nm). To some extent, the absorbance of protein solution at 280 nm is proportional to its concentration, so it can be used for quantitative determination of protein. However, partially purified proteins often contain nucleic acids, and nucleic acids (the maximum absorption peak is 260 nm) can also be absorbed at 280 nm, which will interfere with the determination. Warburg and Christian measured the absorbance (A) ratio of yeast enolase and yeast nucleic acid at 280 nm and 260 nm, and then calculated and corrected the influence of the presence of nucleic acids to obtain the concentration of protein in the presence of nucleic acids. The advantage of this method is that no reagent is added in the quantitative process, and the protein can be recovered.
Warburg Christian formula:
Protein concentration (g/L) =1.55A280 - 0.76A260
The absorbance of 280 nm and 260 nm are multiplied by the coefficient and subtracted to obtain the approximate protein concentration. A280 and A260 represent the absorbance of light with wavelength of 280nm and 260nm when the optical path is 1cm.
1. Main Instruments and Equipment
Micropipette, ultraviolet visible spectrophotometer, quartz cuvette, balance.
2. Experimental Materials
Protein samples extracted by various methods.
3. Main reagents
Sample solution*1
Protein standard solution (1mg/mL): accurately weigh 15mg of bovine serum albumin and prepare 1mg/mL with 15mL of sample solution.
1. Standard Curve Method
(1) Take 8 tubes and add reagents according to Table 2-1-1
Table 2-1-1 Preparation of Protein Standard Curve*2
| Reagent | Test tube number | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Standard solution /mL | - | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 4.0 |
| Sample solution /mL | 4.0 | 3.5 | 3.0 | 2.5 | 2.0 | 1.5 | 1.0 | - |
| Protein content /(mg/mL) | - | 0.125 | 0.25 | 0.375 | 0.5 | 0.625 | 0.75 | 1.0 |
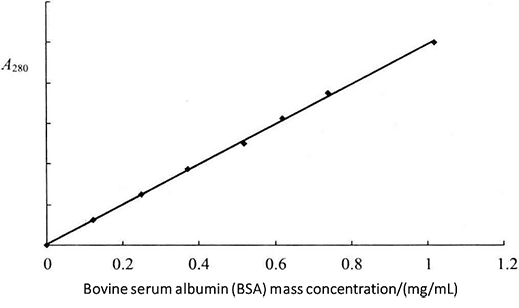 Figure 2-1-1 Standard Curve
Figure 2-1-1 Standard Curve
(2) After mixing, choose a 1cm quartz colorimetric cup*3 and take 3mL of standard solution to measure the absorbance value at 280nm, respectively. First take the solution of tube 1 to adjust the zero point, and then measure the absorbance value of each tube standard separately. Using the absorbance value as the vertical coordinate and the protein concentration as the horizontal coordinate, the standard curve of bovine serum albumin at 280mm was plotted (Figure 2-1-1).
(3) Take an appropriate amount of the protein sample to be measured with the sample solution diluted *4 to 3 mL, mix well, determine the absorbance value according to the above method, and find out the protein concentration according to the standard curve.
2. Warburg-Christian Method
(1) Take an appropriate amount of protein sample to be tested and dilute it n times with the sample solution. Select a quartz cuvette with the optical path of 1cm and measure the absorbance (A) of the solution at the wavelengths of 280nm and 260nm respectively.
(2) The protein concentration of this solution was calculated according to the Warburg-Christian formula and multiplied by the dilution factor n to obtain the true concentration of the protein sample to be measured.
Protein mass concentration (g/L) = (1.55 A280 - 0.76 A280) × n
Waddell and Ressley formula method
In many cases, the contents of tyrosine and tryptophan in different types of proteins (such as serum) are different, and the determination of ultraviolet absorption at 270-290 nm band will have a large change due to the difference in the composition of protein amino acids in each sample, so it is not possible to directly determine the amount of total protein. However, the light absorption of proteins in the far ultraviolet region (200-225 nm) mainly depends on peptide bonds, and all proteins have the same absorption coefficient. With the protein concentration at 120 g/L still in accordance with Beer's Law, Waddell and Ressley et al. established a practical method to determine the total protein in serum at 210 nm wavelength. Therefore, if the protein concentration is within a certain range, A215 and A225 values can be used according to the empirical formula:
Protein mass concentration (g/L) = 144 × (A215 - A225)
(1) Take an appropriate amount of protein sample to be tested and dilute it n times with the sample solution. Select a quartz cuvette with the optical path of 1 cm and measure the absorbance (A) of the solution at the wavelengths of 215 nm and 225 nm respectively.
(2) Calculate the protein mass concentration of this solution according to Waddell Ressley formula, and then multiply by the dilution multiple n to obtain the true content of the protein sample to be tested. Protein mass concentration (g/L) = 144 × (A215 - A225) × n
1. The physicochemical properties of the protein itself and the pH of the buffer solution for dissolving the protein should be considered when determining the concentration. This is because the UV absorption peak of protein changes with the change of pH, which is easy to cause the reading drift.
2. Note the dilution of the sample concentration. Protein absorbance value should be 0.1-1.5. In this range, the interference is relatively small and the result is stable. This means that the concentration of the sample cannot be too low or too high (beyond the test range of the spectrophotometer).
3. Pay attention to the operation. The protein solution should be fully mixed, otherwise the absorbance value is too low, or even negative. The mixed liquid shall be free of bubbles and the blank drop shall be free of suspended solids, otherwise the reading will drift violently. The same cuvette must be used to test the blank solution and sample, otherwise the concentration difference is too large; The conversion factor is consistent with the sample concentration unit. Color cuvettes with worn windows shall not be used. The volume of the sample must reach the minimum volume required by the cuvette.
4. 270-290 nm UV method has certain error for protein solution with large difference between tyrosine and tryptophan content in protein.
5. It is heavily interfered by non-protein factors. In addition to nucleic acid, free tryptophan, tyrosine, uric acid, nucleotide, purine, pyrimidine and bilirubin all interfere.
* 1 This can be distilled water, protein buffer solution or salt solution. Depending on the sample dissolution solution.
* 2 Standard solution preparation.

*3 Quartz colorimetric cup to measure protein concentration.

*4 The concentration should be made to fall within the range of the protein standard curve.


