Principle and Protocol of Isoelectric Focusing Electrophoresis
Isoelectric focusing electrophoresis usually adopts horizontal plate electrophoresis or tube electrophoresis. It has high resolution and sensitivity, and is particularly suitable for studying the microscopic heterogeneity of proteins. For example, a protein shows a single band in SDS-PAGE electrophoresis and three bands in isoelectric focusing. This may be due to the existence of monophosphorylation, diphosphorylation and triphosphorylation of proteins. Since several phosphate groups do not have a significant impact on the relative molecular weight of the protein, they show a single zone in SDS-PAGE gel electrophoresis. However, due to their different charges, they can be separated and detected in isoelectric focusing. There may be only one or two amino acids difference between isozymes, and better separation effect can be obtained by isoelectric focusing. Because proteins are usually in their natural state during isoelectric focusing, enzymes can be detected by active staining. Isoelectric focusing is mainly used for separation and analysis, but it can also be used for purification and preparation. Although the cost is high, the operation is simple and the purification efficiency is high.
Isoelectric focusing can also be used to determine the isoelectric point of an unknown protein. A series of standard proteins with known isoelectric points (usually with a pI of 3.5-10) and the protein to be measured can be isoelectric focused at the same time. Determine the distance from the electrophoresis zone of each standard protein to the edge of one side of the gel and plot the respective pI value to get the standard curve. Then, the distance of the protein to be measured is measured, and its isoelectric point can be calculated through the standard curve.
The earliest isoelectric focusing electrophoresis was vertical plate, and later developed into horizontal plate. Ultra-thin horizontal plate is also developed in recent years. The advantages of this form of electrophoresis are that it saves amphoteric electrolyte reagents, adds more samples, and is conducive to comparing electrophoresis results of different samples. Moreover, the fixation, dyeing and drying after electrophoresis are very convenient and fast. The biggest advantage of horizontal plate isoelectric focusing electrophoresis is that it prevents the drift of pH gradient caused by electroosmosis of electrode solution.
Isoelectric focusing electrophoresis is used to separate amphoteric substances according to their different isoelectric points (pI). Proteins with different isoelectric points of 0.01 can be distinguished. It is an ideal method to separate amphoteric substances such as proteins. The separation principle of isoelectric focusing electrophoresis is to add an amphoteric electrolyte of aliphatic polyamino polycarboxylic acid in the gel. During electrophoresis, the positive electrode solution of the gel plate is phosphoric acid and the negative electrode is sodium chloride oxide. The carrier amphoteric electrolyte carries positive charge in the positive acid environment, but due to the difference of pI, the number of positive charges it carries is different, and the speed of swimming towards the negative electrode is also different during electrophoresis. Similarly, the negative electrode is an alkaline environment, and the amphoteric electrolyte on the carrier carries different amounts of negative charges, swimming towards the positive electrode at different speeds.
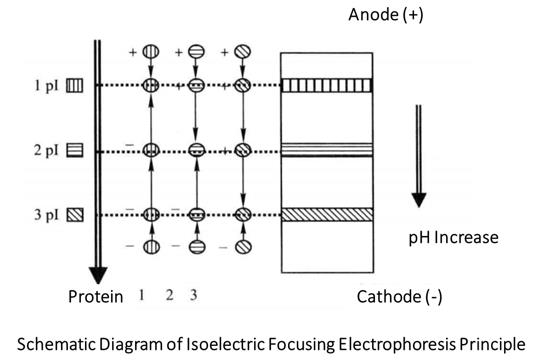
As the amphoteric electrolyte continuously exchanges protons with the solution during swimming, the pH of the solution is changed. When the balance is reached, that is, when the gain and loss of protons are equal, the carrier amphoteric electrolytes reach the isoelectric point and are in their respective pI regions, so the solution also presents different pH, forming a pH gradient with the pl gradient of the carrier amphoteric electrolyte. The pH distribution from anode side to cathode side is linear gradient from low to high, and the linear pH gradient is stable due to the anti-convection and diffusion effect of gel.
When the protein sample is added, the initial charge of the protein depends on the pH of the gel where the sample is placed. The protein with an isoelectric point above pH is positively charged and moves toward the cathode under the action of an electric field. During migration, the pH of the gel where the protein is located gradually increases, and the positive charge of the protein gradually decreases. When reaching the gel area where pH=pI, the protein is not charged and stops migration. Similarly, the protein whose isoelectric point is below the pH value of the gel at the sample loading point is negatively charged and moves towards the anode, and finally stops at the gel area where pH=pI. It can be seen that in the isoelectric focusing process, no matter where the sample is added to the gel, various proteins can move towards their isoelectric points and finally reach their isoelectric points, which has no effect on the final electrophoresis results. So sometimes the sample can be directly added to the gel solution before gel making. Higher voltage (such as 2000 V, 0.5 mm flat gel) can achieve faster separation (0.5-1h), but attention should be paid to cooling the gel and using a constant power supply.
Different amphoteric electrolytes have different pH gradient ranges, ranging from a wide range of pH 3-10 to a narrow range of pH 7-8. Select an appropriate amphoteric electrolyte according to the sample to be separated, so that all components in the sample to be separated are within the pH range of the amphoteric electrolyte. The smaller the pH range of the amphoteric electrolyte, the higher the resolution. When dyeing the protein after the gel is finished, it should be noted that the whole gel will be stained because the amphoteric electrolyte will also be stained. Therefore, the isoelectric focusing gel cannot be dyed directly. It can only be dyed after soaking in 10% trichloroacetic acid to remove the amphoteric electrolyte.
1. Main Instruments and Equipment
Micropipette, stabilized power supply, scanner, balance, water-cooled flat plate electrofocusing electrophoresis tank, glass plate and its fixing device.
2. Experimental Materials
Protein sample with unknown isoelectric point, standard isoelectric focusing sample.
3. Main Reagents
Acrylamide storage solution (30% acrylamide, 3% cross-linking degree): 29.1 g acrylamide and 0.9g methylene bisacrylamide are dissolved in deionized water, with a constant volume of 100mL, filtered out the insoluble matter and stored in a brown bottle, which can be stored for several months at 4 °C.
10% ammonium persulfate solution: Dissolve 0.1 g of ammonium persulfate in 1mL deionized water, and prepare before use. TEMED (Tetramethylethylenediamine), carrier amphoteric electrolyte (pH3.5-10) *1.
Electrode solution: 1mol/L phosphoric acid (anodic solution). The concentration of phosphoric acid reagent is about 16mol/L. When preparing, take 6.75 mL phosphoric acid and add water to 100mL. 1mol/L sodium hydroxide (cathode liquid), weigh 4g solid and dissolve it in 100 mL double distilled water.
Stationary solution: 10 g trichloroacetic acid and 1 g sulfosalicylic acid are dissolved in 80 mL, and the constant volume is 100 mL.
Dye solution: 35mL ethanol, 10mL glacial acetic acid, 0.1g Coomassie brilliant blue R-250, and add water to the volume of 100mL.
Decolorizing solution: 25 mL ethanol, 10 mL glacial acetic acid, and add water to a constant volume of 100 mL.
Sample buffer (5mL): 1.8mL H2O, 200 μL carrier amphoteric electrolyte (the same as the gel making component), 3mL glycerol, and mix with the equal volume sample when loading.
1. Preparation of Gel
Clean two special IEF glass panels, mark the coordinates on the back of the large glass panel, add a few drops of silane reagent on one side of the small glass panel, evenly coat the surface of the glass panel with mirror wiping paper, dry it at room temperature, wash the surface with deionized water, dry it in the air, put a clamp strip between the two glass panels, and clamp it with a clamp.
Glue preparation: 2.0mL monomer storage solution*2, 5.3mL double distilled water, 0.6mL carrier amphoteric electrolyte, add 8 μL TEMED (stock solution) and 10% ammonium persulfate 60 μL. Mix well and inject glue immediately.
2. Sample Loading Electrophoresis
After the gel has solidified, carefully remove the upper glass plate and place the gel on the electrophoresis tank. Dip three layers of filter paper strips 1cm wide and 9cm long into the electrode solution (1mol/L phosphoric acid for anode and 1mol/L sodium hydroxide for cathode), put them on another filter paper to dry the electrode solution on the surface, use tweezers to lay the electrode strips on both ends of the gel, press the electrodes gently to make the electrode strips and adhesive stick tightly, cut off the excess, and use filter paper to absorb the residual liquid on the adhesive surface. According to the coordinate scale on the back of the glass plate, place a small mirror wiping paper at any position on the adhesive surface, add the sample to be tested and the standard sample on the paper, and remember the corresponding coordinate value. Close the cover and turn on the power for electrophoresis.
Start: constant voltage of 60V, after 15min, constant current of 8 mA. The voltage keeps rising during electrophoresis, until the voltage rises to 550V, turn off the power supply.
Open the cover and remove the sample paper, so as to prevent the residual sample on the paper from interfering with the judgment of the result during dyeing.
Adjust the power supply, maintain a constant voltage of 580V, and continue electrophoresis until the current drops to zero (2-3h). After electrophoresis, turn off the power supply.
3. Dyeing and Decolorization
Fixation: take out the gel and put it into the fixative for 15min.
Staining: take out the gel and put it into the staining solution for staining at 65°C for 20min.
Decolorization: take out the gel, wash it with distilled water, decolorize it in the decolorizing solution, shake it constantly, and replace the decolorizing solution for 3-5 times. When the background color is removed*3 and the protein zone is clear, measure and record the length of the gel and the distance from the center of the protein zone to the positive extreme.
3. Preparation of pH Gradient Curve
The swimming distance between the standard isoelectric focusing sample and the unknown sample is calculated according to the coordinate point at the time of sample addition and the distance from the center of each protein zone to the positive extreme.
Plot the pH gradient curve according to the distance and pI value of the known pI protein sample location, with the protein migration distance as the abscissa and the isoelectric point as the ordinate. The isoelectric point can be calculated from the migration distance of the sample.
- Amphoteric electrolyte is the key reagent of isoelectric focusing electrophoresis, and its content is 2% - 3%, which can form a good pH gradient.
- The protein should be salt free. Because of the high salt concentration and large current, it is easy to generate heat, and the salt ions migrate to the two poles to produce acid and alkali, occupying the effective part of the separation. If the sample contains salt ions, the sample tape may be skewed, dragged or not formed at all during electrophoresis.
- The gel of flat isoelectric focusing electrophoresis is very thin. When the current is stable at 8mA and the voltage rises to more than 550V, due to the cathode drift, the local current is too large, and the gel cannot bear it and is burnt off.
- Characteristics of ideal carrier ampholytes:
- The relative molecular weight should be small to separate from the separated macromolecular substances;
- Stable chemical properties;
- The pl of each component is close to each other and has good buffering capacity near its pI value;
- Sufficient conductivity at pl with uniform conductivity;
- Enough amphoteric electrolyte carriers;
- Good solubility.
*1 Amphoteric electrolyte is the key reagent of isoelectric focusing, so special attention should be paid to the quality and quantity of amphoteric electrolyte. The content of amphoteric electrolyte is 2-3%. Good pH gradient can be formed. The carrier amphoteric electrolyte is a mixture formed by the addition reaction of polyvinylamine and acrylic acid. If it is kept for too long, it will grow bacteria, decompose and deteriorate, and cannot be used.
*2 The monomer storage solution is filtered with 0.45μm filter membrane and stored in a dark place at 4 °C. Be careful when using it. The acrylamide contained in it is a nerve agent with accumulation.
*3 Pay attention to the decolorization time, and do not decolorize excessively.


