Guide of Analysis of Transient Cell Interaction
In 1993, Swiss scientist Rossi and others created an agrobacterium-mediated gene transient expression system. They induced agrobacterium with recombinant plasmid to infiltrate plant leaves and then plant cells through vacuum. Then people injected the leaves of living plants through needle tubes to detect the instant expression of genes mediated by Agrobacterium tumefaciens. Now the agrobacterium-mediated gene transient expression conducted by these two methods is collectively referred to as agrobacterium infiltration.
The purpose of this experiment manual is to help scientific researchers understand the principle of cell transient interaction analysis and master the main operating steps and precautions of cell transient interaction analysis.
Cell transient interaction analysis is based on agrobacterium-mediated osmotic transformation, also known as agrobacterium-mediated infiltration method, which uses living tissue cells to detect the protein/nucleic acid interaction instantaneously.
The agrobacterium tumefaciens infiltration method was formed and developed on the basis of the theory that Agrobacterium tumefaciens Ti plasmid-mediated plant cell transformation. It inserts the target gene into the co-integration vector or binary vector to transform Agrobacterium tumefaciens. After the latter is induced by phenolic compounds (such as acetosyrinone), it is injected into the plant leaf tissue through vacuum infiltration or needle tube to transfer T-DNA into the plant nucleus. This method can analyze the binding of promoter and transcription factor in living tissue.
When using firefly luciferase to detect promoter activity, a second reporter gene is usually needed to reduce experimental error. However, traditional auxiliary reporter genes (such as CAT and GUS) are not convenient due to different measurement characteristics. Now we often use the double luciferase reporter gene to detect the promoter activity, and use the sea kidney luciferase as the reporter gene to react the promoter activity according to the ratio of firefly luciferase and sea kidney luciferase.
1. Main Instruments and Equipment
Pipette, micro-centrifuge, 1.5mL centrifuge tube, 50mL centrifuge tube, thermostatic water bath, ice maker, shaking table, oscillator, centrifuge, vacuum meter, ultraviolet spectrophotometer, microplate reader.
2. Material
Tobacco plants, grown at 21°C, 14h light, 10h dark. Agrobacterium infiltration was available when grown to 6 leaves and the youngest leaves were more than 1 cm long.
Agrobacterium GV3101 from pART27-MYB TF, Agrobacterium GV3101 from pART27-bHLH TF, and Agrobacterium GV3101 from pGreen 0800-LUC-DFR promoter.
3. Main Reagents
(1) YEP culture medium
(2) Infiltration buffer: 10 mmol/L MgCl2,0.5 μmol/L acetosyringone
(3) 70% ethanol
(4) PLB (passive lysis buffer)
(5) Dual luciferase assay reagents (Promega company)
1. Preparation of Agrobacterium Bacterium Solution
(1) Strain activation: Agrobacterium stored at -80°C and containing the target gene expression vector was cultured in line on YEP solid medium containing rifampicin and kanamycin.
(2) Pick the monoclonal clone in 3mL YEP liquid medium and incubate overnight at 28°C with 200r/min shaking.
(3) Take 1-1.5mL of bacterial solution into a sterilized 1.5mL centrifuge tube (the amount of bacterial solution can be determined according to the amount to be transformed.)
(4) Centrifuge at 3000 r/min for 10 min at room temperature, discard the supernatant, collect the bacteriophage precipitate, and resuspend with 1 mL of permeate.
(5) Repeat step (3), the residual antibiotics after infiltration may kill the leaf tissue.
(6) Measure the A600 (absorbance value) *1 of the suspension diluted 10 times by UV spectrophotometer.
(7) The final concentration of infiltrate A600 is 0.02-0.2, shaking at room temperature for 2h before infiltration (e.g. overexpression of a protein may kill Agrobacterium, which will cause effects when infiltrating tobacco leaves.)
(8) Generally, about 50-100 μL of each bacterial solution is needed, and the bacteria to be co-transfected into tobacco leaves will be mixed in a 1.5 mL centrifuge tube.
2. Infiltrate Agrobacterium into Tobacco Epidermal Cells
(1) Before Agrobacterium infiltration into tobacco epidermal cells, tobacco plants were placed under white fluorescent light for 1 h to allow tobacco stomata to open fully, which facilitates Agrobacterium infiltration into tobacco epidermal cells.
(2) Select suitable leaves for injection. The third and fourth leaves of the apical meristem of a healthy plant should be selected from the upper part of the plant*2. Two different leaves of the same plant and leaves from different plants should also be selected for replication of the experiment.
(3) Remove the waxy epidermis of the leaf by pricking small holes on the abaxial surface of the leaf with a needle or by rubbing the abaxial surface of the leaf with a syringe (remove the needle) over an area of about 0.5 cm2.
(4) Mark clearly by dividing the area into different areas on the leaf with a marker prior to infiltration.
(5) Use a 1 mL syringe (with the needle removed) to aspirate the suspended Agrobacterium bacterium solution.
(6) Hold the syringe gently against the leaf with your finger and push the syringe slowly against the area marked with the pen on the back of the leaf. Make the bacterium slowly spread to fill the space of the leaf flesh cells. After injection, mark the area where the bacterium spreads with a marker*3.
(7) Put the plant back into normal growth environment.
3. Activity Test of Irefly Luciferase and Renilla Luciferase
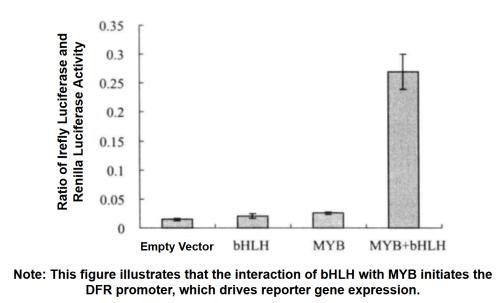
(1) Tobacco leaves were taken separately after 3 d of injection, and the injected area was cut into 2 cm2 pieces (6 replicates for each treatment), and the pieces were put into 500 μL of PLB (passive lysis buffer) for crude extraction.
(2) Add 10 μL of PLB diluted 100 times to the sample, add to 40 μL of luciferase assay buffer, and measure the chemiluminescence value. Stop and add another 40 μL of GlowTM buffer (Promega product) and measure the chemiluminescence value. Relative chemiluminescence values (absolute relative luminescence units) were measured by a Turner 20/20 luminometer (Turner BioSystems Sunnyvale) (5s delay, 15s reading).
1. When conducting tobacco leaf infiltration, care was taken to disrupt the waxy layer on the back of the leaf so that Agrobacterium could penetrate without causing extensive damage to the leaf.
*1 If the A600 absorbance value is much greater than 1.5-2.0, it indicates that a large number of dead Agrobacterium species may be present in the suspension, and this will affect the infiltration efficiency.
*2 The transformation efficiency of young leaves is generally low. Transformation efficiency depends on leaf survival and Agrobacterium infiltration ability. Repeat injections should be made in the same leaf with the same leaf vein.
*3 Be careful to wear gloves, mask and protective glasses when injecting to prevent excessive pressure during injection and spraying of bacterial solution. Wash gloves with 70% ethanol when injecting different strains, and when injecting the same leaf at the same time, the two places of injection should be separated by the leaf veins to avoid cross contamination.


