JAK-STAT Signal Pathway
🧪 KRAS-2569H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-185 aa

🧪 EGF-04H
Source: E.coli
Species: Human
Tag:
Conjugation:
Protein Length:
$149.00
$298
/ 1mg

🧪 EPO-01H
Source: Mammalian Cells
Species: Human
Tag: Non
Conjugation:
Protein Length:

🧪 IL6-12H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 183

🧪 IL10-16H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 160
$72.50
$145
/ 10μg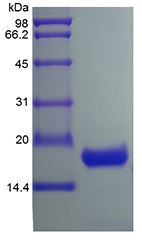
🧪 Il10-17M
Source: E.coli
Species: Mouse
Tag: Non
Conjugation:
Protein Length: 160
$99.00
$198
/ 10μg$224.00
$448
/ 100μg$999.00
$1,998
/ 1mg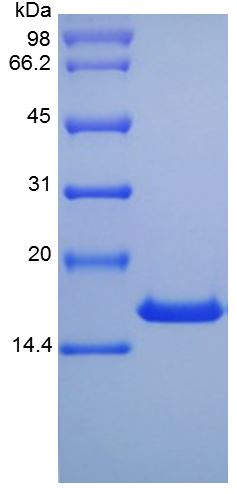
🧪 EPOR-28H
Source: Human Cells
Species: Human
Tag: Fc
Conjugation:
Protein Length: 1-250 a.a.

🧪 EGFR-30H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: 1-645 aa
🧪 IFNB1-14H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: 1-187 a.a.
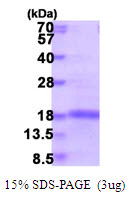
🧪 Il2-106M
Source: E.coli
Species: Mouse
Tag: Non
Conjugation:
Protein Length: 21-169 a.a.

🧪 HDAC5-396H
Source: Insect Cells
Species: Human
Tag: GST
Conjugation:
Protein Length:
🧪 IFNGR1-22H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: 1-245 a.a.
🧪 IL2-116H
Source: HEK293
Species: Human
Tag: Non
Conjugation:
Protein Length:
🧪 IL3-120H
Source: HEK293
Species: Human
Tag: Non
Conjugation:
Protein Length:

🧪 IL3RA-121H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: Thr19-Arg305
Background of Hippo Signal Pathway
What is JAK-STAT Signal Pathway?
JAK-STAT signal pathway is a cytokine-stimulated signal transduction pathway found in recent years. It is involved in many important biological processes such as cell proliferation, differentiation, apoptosis and immune regulation. Compared with other signaling, the JAK-STAT signal pathway is relatively simple. It consists of three major components: receptor tyrosine kinase (RTK), Janus kinase (JAK) and signal transducer and activator of transcription (STAT).
When the ligand binds to the receptor, JAKs is activated and phosphorylates the intracellular domain of the receptor, which in turn phosphorylates STATs. Phosphorylated STATs is then dimerized and translocated into the nucleus to regulate the transcription of the target gene. This pathway plays a key role in many physiological processes, including immune response, cell proliferation, differentiation, and response to extracellular stimuli.
Core Components of JAK-STAT Signal Pathway
RTK
Many cytokines and growth factors signal through the JAK-STAT signal pathway, including interleukin 2/7 (IL-2/IL-7), granulocyte/macrophage colony stimulating factors (GM-CSF), growth hormones (GH), epidermal growth factors (EGF), platelet-derived growth factors (PDGF), interferons (IFN) and so on (Figure 1). These cytokines and growth factors have corresponding receptors on the cell membrane. These receptors belong to the cytokine receptor superfamily and can be classified into two groups: Type I, the extracellular portion contains four conserved cysteines and one WSXWS unit at the C-terminal. Type II, the N-terminal and C-terminal of the extracellular part have a few pairs of cysteine, respectively. A common feature of these RTKs is that they does not have kinase activity, but the intracellular domain has a binding site for the tyrosine kinase JAK. After binding to the ligands, receptors activate JAK. Tyrosine residues of various target proteins are phosphorylated by activation of JAK to effect signal transduction from the extracellular to the intracellular.
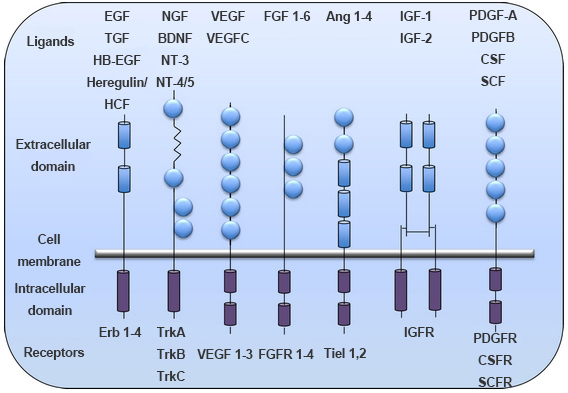
Figure 1. Different classes of RTKs. (Vigneswara V; et al. 2012)
The structure and function studies of RTKs showed no obvious homology. There is only a homologous region in the cytoplasmic near membrane region, which was a functional segment that bound to JAK. This homology region usually consists of two structures that are highly conserved among various receptors, one of which is the prolyl-rich "box1" and the other is the "box2" near the cell membrane, which are the most important structure that determines the mutual coupling between a cytokine receptor and a JAK kinase. The dimerization of receptors can be homologous or heterologous. In the dimerization of homologous receptor, only JAK2 is activated. In contrast, heterodimers consisting of different subunits activate a wide range of JAKs. Once activated, JAKs phosphorylate receptor subunits and other substrates.
JAK
Many tyrosine kinases are cell membrane receptors, collectively referred to as RTKs, whereas JAKs are a class of non-transmembrane tyrosine kinases that have only catalytic domains but no Src homology 2 (SH2) domain. The JAK protein family consists of four members: JAK1, JAK2, JAK3 and Tyk2, which have seven JAK homology (JH) domains. The JH1 domain is a kinase domain and the JH2 domain is a pseudo-kinase domain, JH6 and JH7 are receptor binding regions (Figure 2). For example, in JAK3, JH1 is the C-terminal tyrosine kinase domain. Adjacent to the kinase domain is the JH2, or pseudo-kinase domain, which itself lacks catalytic activity but is essential for regulating normal kinase activity. The amino terminus of the JAKs (JH5–JH7) has homology to the band four-point-one, ezrin, radixin, moesin (FERM)-domain containing proteins. This region of the JAKs mediates binding to cytokine receptors and also regulates catalytic activity. Mutations have been identified in all of these domains; most have considerable effects on the protein expression of JAKs, but some missense mutations or small in-frame deletions allow for some protein expression. These mutations affect kinase activity, receptor binding and intracellular transport.
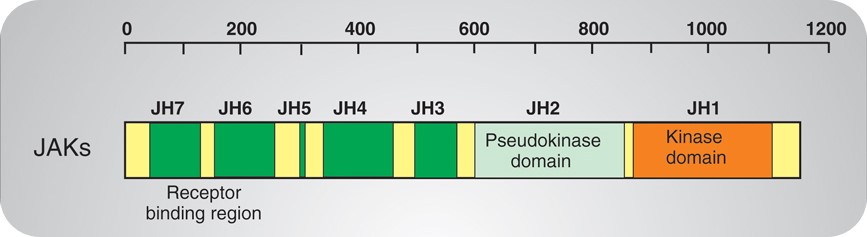
Figure 2. Structure of JAKs.
STAT
STAT is called "signal transducer and activator of transcription." As its name implies, STATs play a key role in signal transduction and transcriptional activation. Since the first STAT protein STAT1 was purified in 1991, other members of the STAT protein family have also been cloned. Currently, six members of the STAT family have been found, STAT1, STAT2, STAT3, STAT4, STAT5, and STAT6. STAT proteins are structurally divided into the following functional segments: N-terminal conserved sequence, DNA binding domain, SH3 domain, SH2 domain and C-terminal transcriptional activation domain (Figure 3). Among them, the most conserved and functionally most significant sequence is the SH2 domain, which has the exact same core sequence "GTFLLRFSS" as the SH2 domain.
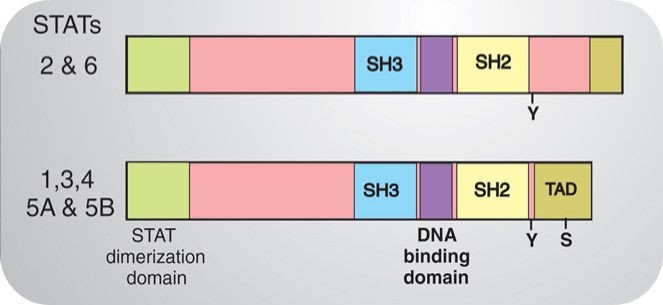
Figure 3. Structure of STATs.
JAK-STAT Signal Pathway Related Diseases
Dysregulation of the JAK-STAT signaling pathway has been associated with the development of a variety of diseases, including but not limited to immune deficiency syndrome, cancer, and inflammatory diseases. In the field of cancer, abnormal activation of the JAK-STAT pathway is associated with the proliferation, survival, and metastasis of tumor cells, especially in leukemia, lymphoma, and a variety of solid tumors. In addition, overactivation of this signaling pathway is also associated with autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus. In infectious diseases, abnormalities in the JAK-STAT pathway may also lead to an imbalance in the immune response and affect the course of the disease.
Case Study
Case Study 1: Recombinant Human STAT3 (STAT3-1496H)
The gene that encodes the epidermal growth factor receptor (EGFR) is frequently overexpressed or mutated in human cancers, including glioblastoma. However, the efficacy of EGFR-targeted small-molecule inhibitors or monoclonal antibodies in glioblastomas that also have mutation or deletion of the gene encoding phosphatase and tensin homolog (PTEN) has been modest. The researchers found that EGFR signaling was blocked by a small molecule (G5-7) that selectively inhibited Janus kinase 2 (JAK2)-mediated phosphorylation and activation of EGFR and STAT3 (signal transducer and activator of transcription 3) by binding to JAK2, thereby decreasing the activity of downstream signaling by mTOR (mammalian target of rapamycin) and inducing cell cycle arrest. G5-7 inhibited the proliferation of PTEN-deficient glioblastoma cell lines harboring a constitutively active variant of EGFR (U87MG/EGFRvIII) and human glioblastoma explant neurosphere cultures, but the drug only weakly inhibited the proliferation of either glioblastoma cell lines that were wild type for EGFR and stably transfected with PTEN (U87MG/PTEN) or normal neural progenitor cells and astrocytes.
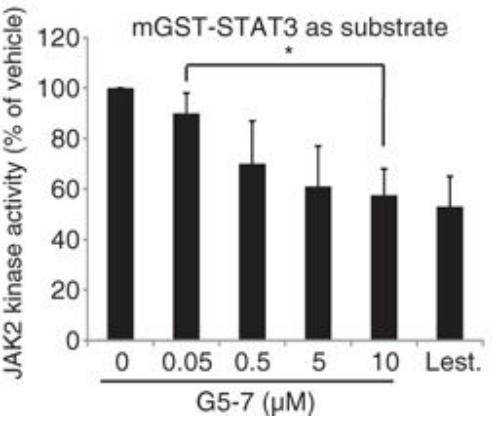
Fig1. Analysis of the in vitro kinase activity of JAK2. (Kunyan He, 2013)
Case Study 2: Recombinant Chicken CSF2 protein (CSF2-16C)
Hematopoietic stem and progenitor cell (HSPC) research will help elucidate the pathogenesis of hematologic diseases. The present study aimed to establish an isolation method and culture system for chicken bone marrow (BM)-derived HSPCs and test their proliferation and differentiation abilities. Mononuclear cells were collected from chicken BM, and CD34+ HSPCs were isolated. Then, the cells were cultured in media with different cytokine compositions, and the growth status, cell phenotype, and morphological appearance of the cells were analyzed at different time points. Our results showed that Iscove's Modified Dulbecco's Medium supplemented with 50 ng/mL stem cell factor, 30 ng/mL Flt-3 ligand, 10 μg/mL interleukin 3, 50 ng/mL interleukin 6%, and 10% chicken serum supported chicken CD34+ HSPC survival ex vivo for approximately 10 d. Further, 80 ng/mL granulocyte-colony stimulating factor and 30 ng/mL granulocyte macrophage-colony stimulating factor were added into the above culture system to form a myeloid cell differentiation induction culture system. After culturing in this system for 72 h, approximately 66% of chicken CD34+ HSPCs exhibited a CD11b+ phenotype, indicating that HSPCs differentiated into myeloid cells.
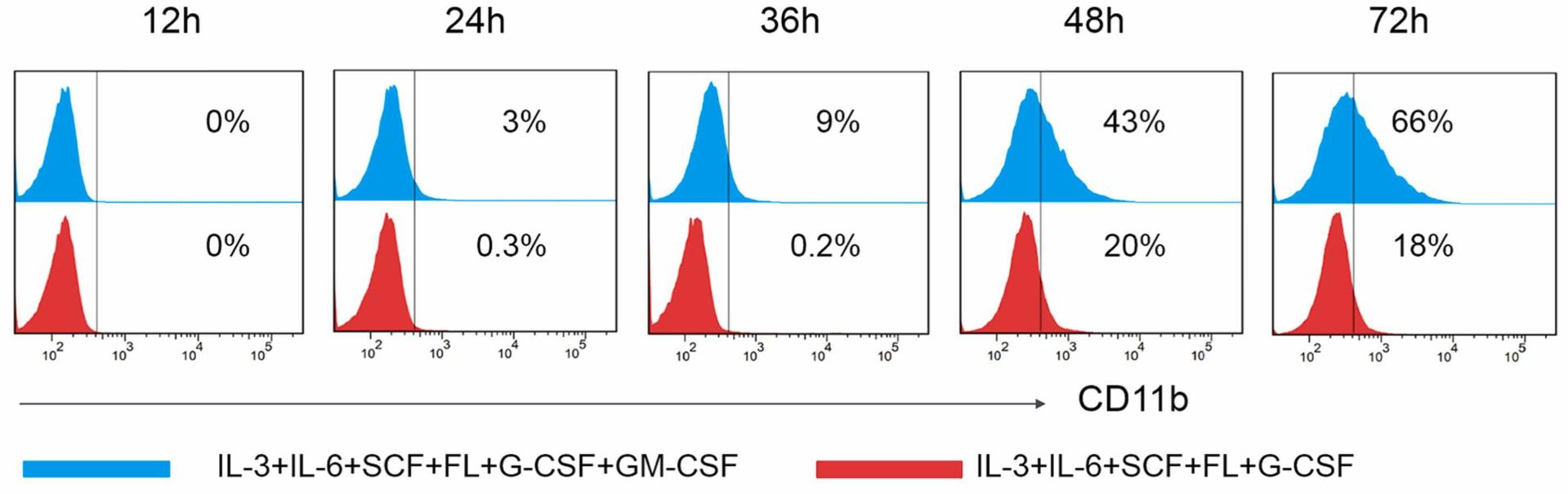
Fig2. Flow cytometric analysis of immunophenotype CD11b+ myeloid cells. (Shuhai He, 2023)
Case Study 3: Recombinant Human Histone Deacetylase 1 (HDAC1-392H)
Gefitinib is an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR TKI) for treating advanced non-small cell lung cancer (NSCLC). However, drug resistance seriously impedes the clinical efficacy of gefitinib. This study investigated the repositioning of the non-oncology drug capable of inhibiting histone deacetylases (HDACs) to overcome gefitinib resistance. A few drug candidates were identified using the in silico repurposing tool "DRUGSURV" and tested for HDAC inhibition. Flunarizine, originally indicated for migraine prophylaxis and vertigo treatment, was selected for detailed investigation in NSCLC cell lines harboring a range of different gefitinib resistance mechanisms (EGFR T790M, KRAS G12S, MET amplification, or PTEN loss). The acetylation level of cellular histone protein was increased by flunarizine in a concentration- and time-dependent manner. The gefitinib-flunarizine combination was shown to induce the apoptotic protein Bim but reduce the antiapoptotic protein Bcl-2, which apparently circumvented gefitinib resistance. The induction of Bim by flunarizine was accompanied by an increase in the histone acetylation and E2F1 interaction with the BIM gene promoter. Flunarizine was also found to upregulate E-cadherin but downregulate the vimentin expression, which subsequently inhibited cancer cell migration and invasion.
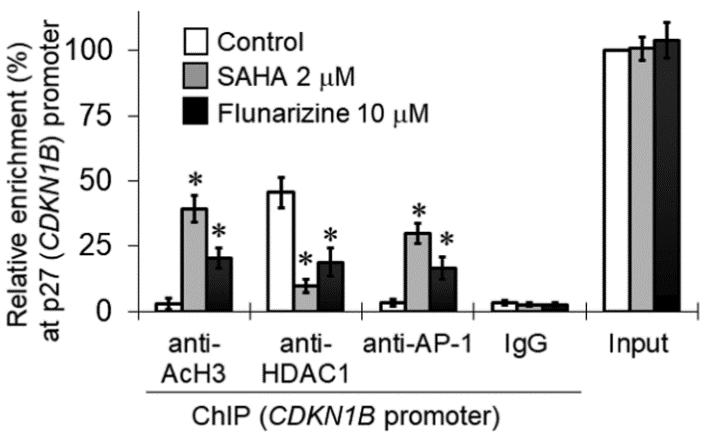
Fig3. Flunarizine induced p27Kip1 expression in H1975 cells. (Kenneth K W To, 2023)
Related Products
JAK-STAT signal pathway is a cytokine-stimulated signal transduction pathway which consists of three major components: RTK, JAK, and STAT. Although there is ample evidence that sustained activation of the JAK-STAT signal pathway is closely linked to diseases, the development of diseases is the result of multiple genetic anomalies that involve multiple steps. Creative BioMart can provide a list of core protein products of the JAK-STAT signal pathway to help you design specific inhibitors against these proteins and bring new hope for the treatment of the diseases. Please feel free to contact us if you’re interested.


