Dendritic Cell Development
Available Resources for the Study of Dendritic Cell Development
We understand the importance of dendritic cell development research and the need for reliable and accurate products and solutions. That is why we have dedicated our resources to ensuring that researchers have access to the best tools available. Our products include recombinant proteins and others.
In addition to our high-quality products, we also offer personalized solutions. Our team of experts is available to answer any questions or concerns you may have and to provide guidance on experimental design and protocols. We understand that each researcher's needs are unique, and we strive to tailor our solutions to meet those specific requirements.
Furthermore, our comprehensive collection of resources is designed to support your research journey. These resources are carefully curated to provide in-depth information on dendritic cell development, including involved pathways, protein functions, and interacting proteins.
At Creative BioMart, our ultimate goal is to contribute to the advancement of dendritic cell development research. We are committed to providing exceptional products, personalized solutions, and extensive resources to support your scientific endeavors. Trust us to be your reliable partner in your research journey.
Our Featured Products
- Recombinant Human CD83 protein, His-tagged
- Recombinant Mouse Cd83 protein, hFc-tagged
- Active Recombinant Human CD40, Fc Chimera
- Recombinant Human CD40, Fc-His tagged
- Recombinant Full Length Human CD40 Protein, GST tagged
- Recombinant Rat Cd40 protein, hFc-tagged
- Recombinant Rhesus CD40 protein, His-tagged
- Active Recombinant Human CD80, His-tagged, Biotinylated
- Recombinant Mouse Cd80 Protein, His-tagged, FITC conjugated
- Recombinant Cynomolgus/Rhesus macaque CD80 protein, Fc-tagged
- Active Recombinant Human CD86 Protein, His & Avi-tagged, Biotinylated
- Recombinant Human CD86, GST-tagged
- Recombinant Rat Cd86 Protein, His-tagged, FITC conjugated
- Active Recombinant Human CSF1 protein, His-tagged
- Recombinant Chicken CSF1 Protein
- Recombinant Human CSHL1, GST-tagged
- Recombinant Human IRF8, His-tagged
- Recombinant Human NOTCH2 protein, His-tagged
- Recombinant Human STAT3, GST-tagged
If you have any questions, requirements, or cooperation intentions, please feel free to contact us . We very much look forward to working with you and helping you achieve research and commercial success.
About Dendritic Cell Development
Dendritic cell (DC) development is a complex and dynamic process that occurs in the bone marrow and peripheral tissues. DCs are a specialized group of immune cells that play a critical role in initiating and modulating immune responses. Here's an introduction to dendritic cell development:
- Hematopoietic Stem Cell (HSC) Differentiation: Dendritic cells are derived from hematopoietic stem cells in the bone marrow. HSCs give rise to common myeloid progenitors (CMPs) and common lymphoid progenitors (CLPs). DCs primarily develop from the myeloid lineage.
- Commitment to the Dendritic Cell Lineage: CMPs differentiate into more restricted progenitor cells known as common dendritic cell progenitors (CDPs). CDPs are committed to the dendritic cell lineage and possess the potential to differentiate into different subsets of DCs.
- Differentiation into Precursor Populations: CDPs migrate from the bone marrow to peripheral tissues, where they further differentiate into precursor populations. There are two major subsets of dendritic cell progenitors: conventional dendritic cell progenitors (CDPc) and plasmacytoid dendritic cell progenitors (CDPp).
- Conventional Dendritic Cell Development: CDPc undergoes further differentiation and maturation to give rise to conventional dendritic cells (cDCs). This process involves the acquisition of specific transcription factors, such as IRF8, Batf3, and Id2, which are critical for cDC development. cDCs can be further classified into different subsets based on their expression of specific markers, such as CD8α+ cDCs and CD11b+ cDCs in mice.
- Plasmacytoid Dendritic Cell Development: CDPp differentiate into plasmacytoid dendritic cells (pDCs). The development of pDCs involves the expression of specific transcription factors, including E2-2 (TCF4), Spi-B, and IRF8, along with the upregulation of cell surface markers, such as CD123 and CD303.
- Maturation and Activation: Once dendritic cells have developed, they undergo maturation and activation in response to environmental cues, such as pathogen-derived signals or inflammatory cytokines. Maturation involves changes in cell surface marker expression, upregulation of co-stimulatory molecules (e.g., CD80, CD86), and increased antigen-presenting capacity.
- Migration to Peripheral Tissues and Lymphoid Organs: Mature dendritic cells migrate from peripheral tissues to secondary lymphoid organs, such as lymph nodes or spleen. This migration is essential for their encounter with naïve T cells and initiation of immune responses.
- Antigen Capture and Presentation: Dendritic cells are specialized in capturing antigens from their surroundings, processing them into smaller fragments, and presenting them on their cell surface using major histocompatibility complex (MHC) molecules. This process enables dendritic cells to present antigens to T cells and initiate adaptive immune responses.
Understanding the development of dendritic cells is crucial for unraveling their roles in immune surveillance, immune tolerance, and the initiation of immune responses. Further research in this field holds promise for developing novel immunotherapies, vaccines, and strategies to modulate immune responses in various diseases.
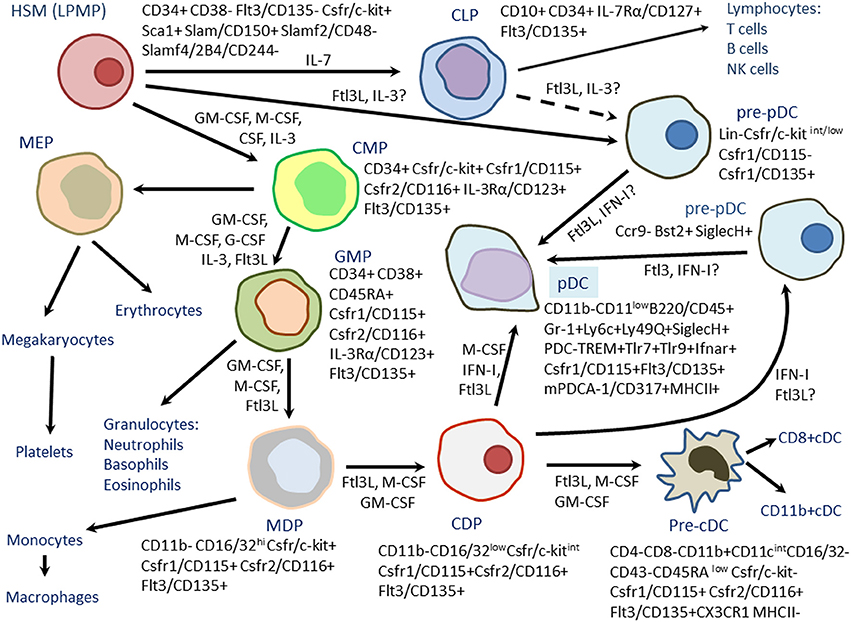 Fig.1 Differentiation of mouse plasmacytoid dendritic cells (PDCs) from hematopoietic stem cells (HSCs) Or lymphoid-primed multipotent progenitors (LPMPs). (Boudreau J E, et al., 2011)
Fig.1 Differentiation of mouse plasmacytoid dendritic cells (PDCs) from hematopoietic stem cells (HSCs) Or lymphoid-primed multipotent progenitors (LPMPs). (Boudreau J E, et al., 2011)
Regulatory Mechanisms Affecting Dendritic Cell Development
The development of dendritic cells (DCs) is tightly regulated by various factors and signaling pathways. Here are some key regulatory mechanisms that affect dendritic cell development:
- Transcription Factors: Transcription factors play a crucial role in regulating the differentiation and maturation of dendritic cells. Different subsets of DCs are characterized by the expression of specific transcription factors. For example, the transcription factor PU.1 is essential for the commitment of common myeloid progenitors (CMPs) to the DC lineage. IRF8, Batf3, and Id2 are involved in the development of conventional dendritic cells (cDCs), while E2-2 (TCF4) and Spi-B are critical for plasmacytoid dendritic cell (pDC) development.
- Cytokines: Cytokines are signaling molecules that profoundly influence dendritic cell development. Granulocyte-macrophage colony-stimulating factor (GM-CSF) promotes the development of DCs, particularly cDCs, by expanding the common dendritic cell progenitors (CDPs). Flt3 ligand (Flt3L) is crucial for the development of both cDCs and pDCs and promotes the expansion and differentiation of early DC progenitors. Interferon-alpha (IFN-α) and interleukin-3 (IL-3) are involved in pDC development.
- Notch Signaling: Notch signaling pathway is involved in the development and differentiation of multiple cell types, including dendritic cells. Notch receptors and ligands are expressed on DC progenitors and regulate their fate decisions. Notch signaling influences the commitment of CMPs to the DC lineage and promotes the development of cDCs.
- Microenvironmental Factors: The local microenvironment and tissue-specific signals play a critical role in shaping dendritic cell development. Factors such as stromal cells, extracellular matrix components, and chemokines present in specific tissues influence the migration, survival, and differentiation of DC progenitors.
- Toll-like Receptor (TLR) Signaling: Toll-like receptors are a family of pattern recognition receptors that recognize pathogen-associated molecular patterns (PAMPs). Activation of TLR signaling in dendritic cells induces their maturation and enhances their antigen-presenting capacity. TLR signaling also influences the development and differentiation of dendritic cell progenitors.
- Negative Regulators: Several negative regulators modulate dendritic cell development to maintain immune homeostasis and prevent excessive immune responses. Factors such as IL-10 and transforming growth factor-beta (TGF-β) can inhibit dendritic cell development and maturation, promoting tolerance and immune regulation.
- Epigenetic Regulation: Epigenetic modifications, such as DNA methylation and histone modifications, play a role in regulating dendritic cell development. These modifications can influence gene expression patterns and determine the fate of DC progenitors.
Understanding the regulatory mechanisms that govern dendritic cell development is crucial for manipulating their function and optimizing immune responses in various diseases. Manipulating these factors and pathways holds promise for developing novel immunotherapies, vaccines, and strategies to modulate immune responses in conditions such as infections, cancer, and autoimmune disorders.
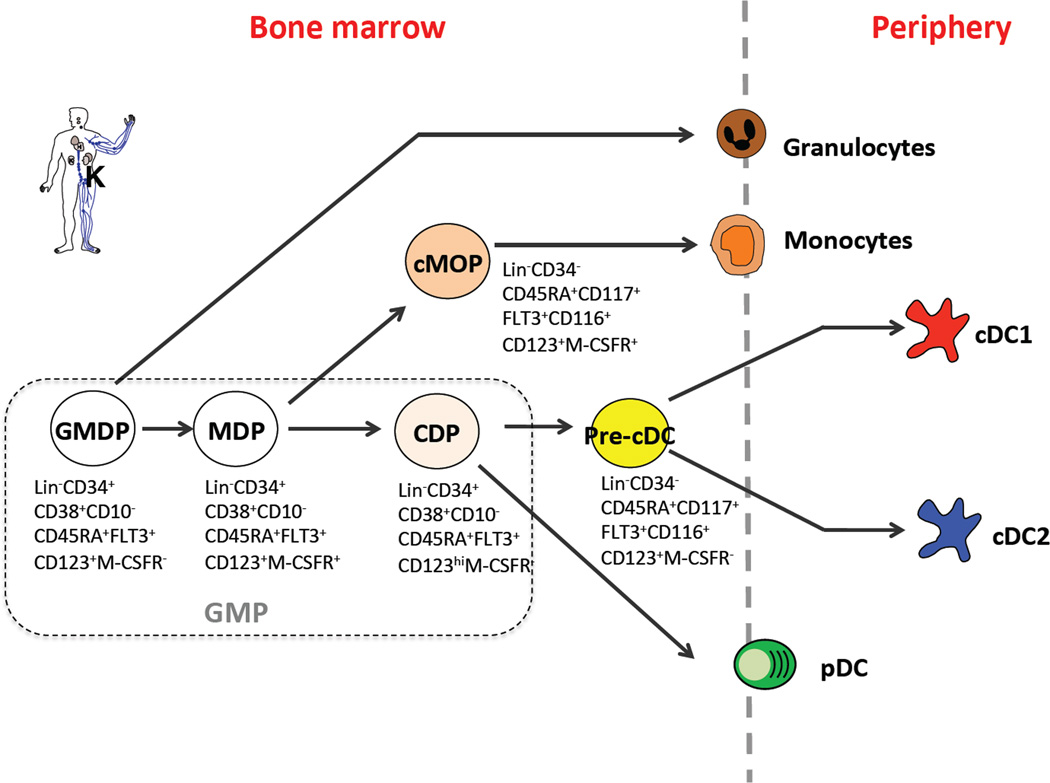 Fig.2 Phenotype and relationship of DC progenitors in human. (Puhr S, et al., 2015)
Fig.2 Phenotype and relationship of DC progenitors in human. (Puhr S, et al., 2015)
References:
- Bieber K, Autenrieth S E. Dendritic cell development in infection[J]. Molecular immunology, 2020, 121: 111-117.
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563-604. doi:10.1146/annurev-immunol-020711-074950
- Anderson DA 3rd, Dutertre CA, Ginhoux F, Murphy KM. Genetic models of human and mouse dendritic cell development and function. Nat Rev Immunol. 2021;21(2):101-115. doi:10.1038/s41577-020-00413-x
- Puhr S, Lee J, Zvezdova E, Zhou YJ, Liu K. Dendritic cell development-History, advances, and open questions. Semin Immunol. 2015;27(6):388-396. doi:10.1016/j.smim.2016.03.012


