Uncategorized Saturday, 2025/08/23
This study established a patient-derived organoid model of head and neck squamous cell carcinoma (HNSCC) using a WNT-free culture medium. The organoids closely mimic the primary tumor characteristics and can predict the patients' responses to chemoradiation therapy, thus providing a powerful tool for personalized treatment.
Head and neck squamous cell carcinoma (HNSCC) is a prevalent and lethal malignant tumor with significant treatment-related toxicity. According to the American Cancer Society, there will be over 66,000 new cases of oral, pharyngeal, and laryngeal cancer in the United States in 2023, with over 15,000 deaths. These cancers exhibit strong molecular heterogeneity, with more than 95% being squamous cell carcinomas. They often present with inactivation of tumor suppressor genes such as TP53 and CDKN2A, and activation of oncogenes like EGFR and HRAS, leading to genetic abnormalities. The median survival time for patients with recurrence or metastasis is only 14.9 months, and most have resistance to chemoradiation therapy.
Our Related Proteins
To address this clinical challenge, a study published in Sci Rep titled "Head and neck tumor organoid grown under simplified media conditions model tumor biology and chemoradiation responses" developed a patient-derived organoid (PDO) model featuring WNT-free culture media as its core technical highlight. Unlike traditional mediums containing Wnt agonists, this model avoids the use of Wnt3a or CHIR99021 due to the significantly lower Wnt pathway activation in HNSCC compared to colorectal cancer. This approach maintains the tumor differentiation state, reduces stem cell-like phenotypes, and preserves tumor heterogeneity. The medium contains bovine pituitary extract, EGF, FGF, and other components that support organoid growth while inhibiting fibroblast over-proliferation.
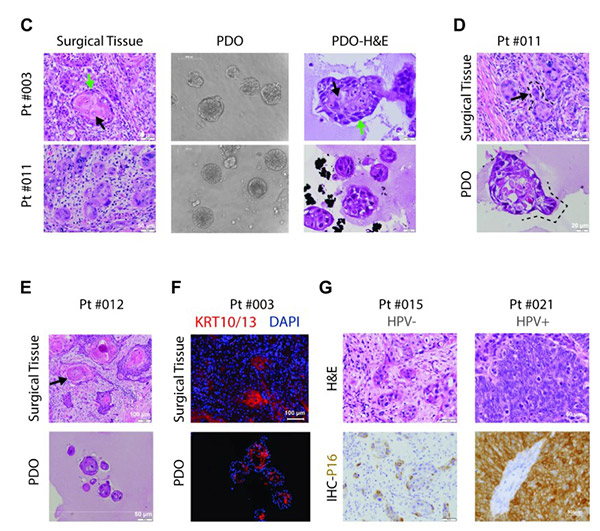
Fig1. Morphological and pathological recapitulation of patient-derived organoids with matched tumor sources.
The study recruited 51 patients and processed 34 tumor tissues, achieving a PDO establishment success rate of over 40% with the criterion for success being the ability to pass for four or more times. PDOs showed a high level of consistency with primary tumors in various aspects. Histopathologically, they replicated features like tumor cell nest structures and keratin pearls. For example, PDO from Pt#003 retained the layered formation of basal-like and mature cells from the primary tumor. At the molecular level, the p16 expression status matched the HPV infection status, and the expression of markers like CD44 and KRT5 was consistent. Genomic analysis through whole-exome sequencing showed a high similarity in SNVs and CNVs between PDOs and primary tumors, sharing key gene mutations such as TP53.
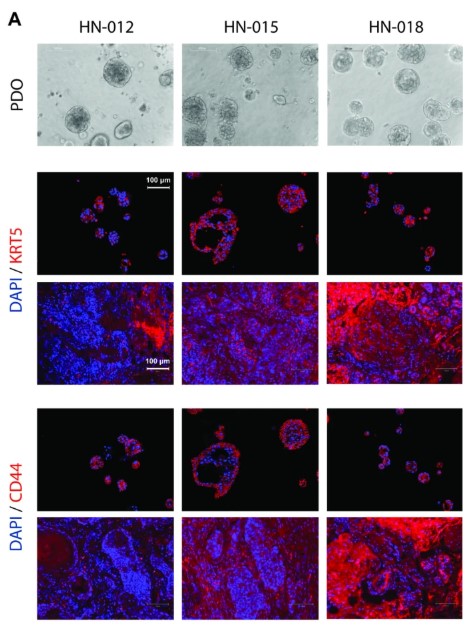
Fig2. Cellular Biological Characteristics of Patient-derived Organoid Models
In terms of predicting treatment response, PDOs exhibited patient-specific reactions. During radiation testing, HN-011 was sensitive to 8Gy radiation with a significant decrease in survival, whereas HN-003 showed no noticeable change. In chemotherapy testing, HN-011 showed the highest sensitivity to cisplatin and carboplatin, correlating with the clinical outcome of 34 months disease-free survival post-chemoradiation therapy for the patient, whereas HN-003's PDO showed radiation resistance, aligning with the patient's survival outcome of death after 19 months post-treatment.
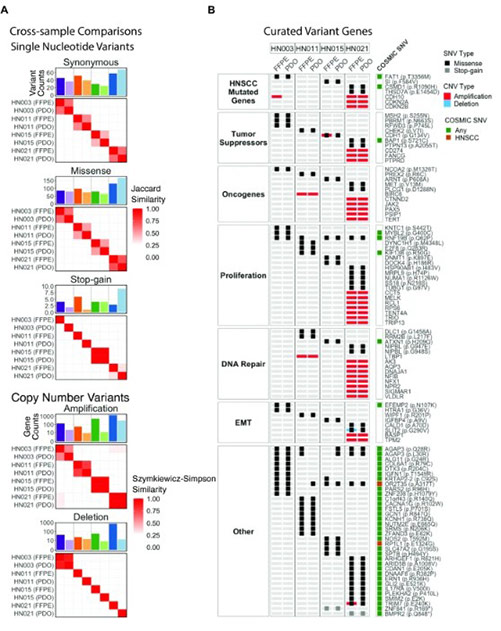
Fig3. Genetic concordance between PDOs and surgical tissue FFPEs revealed by whole exome sequencing.
This PDO model offers a new tool for HNSCC research, useful in understanding pathogenesis, screening new drugs, and promoting personalized treatment. It holds great potential in minimizing treatment toxicity and optimizing treatment regimens, potentially paving the way for improved patient prognoses.
Related Products & Services
- Cytokines for Organoid Culture
- ADC Target Protein
- PROTAC Targets
- Cell and Gene Therapy
- Targets of CAR-T Cell Therapy
- Cancer Drug Targets
- Immune Checkpoint Proteins
- Protein Engineering Services
- Protein Interaction Service
- Protein Expression and Purification Services
- Drug Discovery Screening
- Protein Pathway Profiling
Reference
- Li W, Nishino M, Reed E, et al. Head and neck tumor organoid grown under simplified media conditions model tumor biology and chemoradiation responses. Sci Rep. 2025;15(1):24221. Published 2025 Jul 7. doi:10.1038/s41598-025-88082-5

