Brief Introduction
Vincent Allfrey and his colleagues first identified lysine acetylation on histones in 1964. Acetylation is an evolutionarily conserved post-translational modification (PTM) that is present in both prokaryotes and eukaryotes. Histone acetylation and deacetylation are catalyzed by histone acetyltransferases (HATs) and histone deacetylases (HDACs), respectively. HAT and HDAC-catalyzed acetylation play an important role in the regulation of eukaryotic gene expression. These two kinds of enzymes regulate the acetylation level of core histones through reversible modification of core histone, thus regulating the initiation and elongation of transcription. In general, histone acetylation promotes transcription, while deacetylation inhibits transcription.
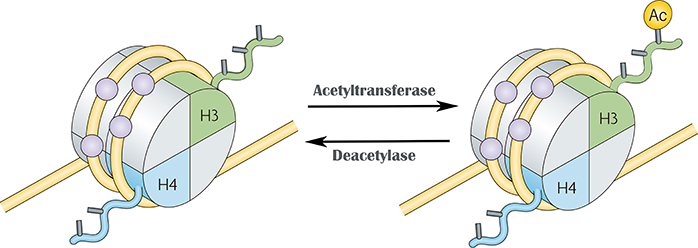
An increasing number of studies have identified many non-histone acetylation modifications and their important biological functions. Acetylation modification affects protein function through a variety of mechanisms, including regulation of protein stability, enzymatic activity, subcellular localization, protein-protein interactions, protein-DNA interactions, etc.
Creative BioMart offers a variety of reliable HATs, HDACs, and proteins/protein complexes associated with histone/non-histone acetylation.
USA
Enter your email here to subscribe.
Follow us on

Easy access to products and services you need from our library via powerful searching tools