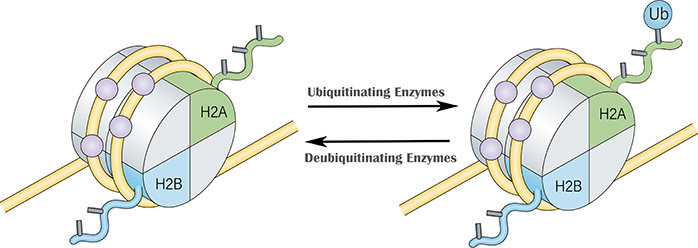
Protein ubiquitination is the process by which the carboxyl-terminus of the ubiquitin molecule binds to the lysine residue of the protein. There are three types of enzyme catalysis in the ubiquitination regulatory pathway: ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2) and ubiquitin-protein ligase, E3). Polyubiquitination requires the interaction of the above three enzymes, while monoubiquitination generally requires only the first two enzymes. Histone H2A and histone H2B can be modified by ubiquitination, for example, two common ubiquitination sites are H2A Lys119 and H2B Lys120. In contrast, histone H1 and H3 have fewer ubiquitinated forms than H2A and H2B. Histone ubiquitination is also a reversible regulation. The dynamic equilibrium process of histone ubiquitination is determined by two factors: free ubiquitin available in the cell, and the activity of histone ubiquitinating enzymes or deubiquitinating enzymes. Histone ubiquitination requires a series of enzymes E1, E2, and E3, while histone deubiquitination requires the action of peptidase.
The histone SUMOylation is a recently discovered histone modification. SUMO (small ubiquitin-related modifier) is a member of the ubiquitin-like protein family and is capable of covalently binding to the lysine residue of histones. The SUMOylation of histones can also regulate gene transcription and affect the occurrence and development of certain diseases.
Creative BioMart provides high-quality proteins and enzymes associated with histone ubiquitination and SUMOylation to speed up your epigenetic research.
Ubiquitination & SUMOylation Proteins Products ListUSA
Enter your email here to subscribe.
Follow us on

Easy access to products and services you need from our library via powerful searching tools