Phosphorylation of histone at serine or threonine is highly conserved in eukaryotes and shows influences on chromatin condensation during mitosis and transcriptional activation of immediate-early genes, DNA repair and apoptosis as well as cell growth. In most species, phosphorylation of H3 at Ser10, Ser28, Thr3, and Thr11 is a primary modification. Histone H3 phosphorylation at Ser10 regulated by the cell cycle has been utilized as a mitotic marker and is critical for neoplastic cell transformation. Several protein kinases, such as aurora B, proton pump inhibitor, and protein kinase C, account for histone phosphorylation, and their inhibition or activation could result in an alteration in intracellular histone phosphorylation. It is known that many diseases are caused by abnormal phosphorylation, while some phosphorylation is the result of a disease. Quantitative detection of the phosphorylation level in histone would afford valuable information for better comprehension of epigenetic regulation of cellular processes together with pathological process of some diseases, and for the development of protein kinase-targeted drug.
To meet your specific needs in epigenetics, Creative BioMart is proud to provide convenient, trusted and custom set of solutions for phosphorylated histone quantification at multiple sites through a comprehensive service list, which covers all essential procedures needed for measuring histone phosphorylation.
What Is Phosphorylated Histone Quantification (PHQ) Assay?
Histone phosphorylation plays critical roles in chromatin remodeling associated with other nuclear processes. PHQ assay is a whole cell-based detection protocol and is intended for in situ quantifying total degrees of histone phosphorylation or specifically individual levels of amino acid phosphorylation based on a colorimetric or fluorometric technique. It is suitable for measuring phosphorylated histone from human, mouse, and rat samples, including fresh and frozen tissues as well as cultured adherent and suspension cells.
Procedure of PHQ Assay
In PHQ assay, adherent cells cultured in conventional 96-well microplates are treated, fixed and permeabilized, sequentially. The phosphorylated histone is then captured to the strip plates coated with a capturing antibody, which can subsequently be recognized with a detection antibody. A color development reagent is then added. The ratio of phosphorylated histone is proportionate to the absorbance intensity and the absolute amount histone can be quantified through HRP conjugated secondary antibody-color development system in comparison to the standard control.
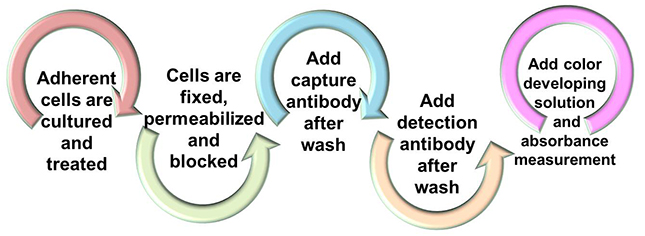
Figure 1. Workflow of PHQ Assay.
Features of Our PHQ Assay
With state-of-the-art platforms and in-depth collaboration with experienced technicians in Creative BioMart, our PHQ assay service is reliable to satisfy each demand from our customers with increased efficiency and reduced cost. We hope to collaborate with you to explore new frontiers in biological science and drive your research on the accelerated track of success. If you have additional requirements or questions, please feel free to contact us.
Reference
1. Rossetto, D.; et al. Histone phosphorylation: A chromatin modification involved in diverse nuclear events. Epigenetics. 2012, 7(10):1098–1108.
USA
Enter your email here to subscribe.
Follow us on

Easy access to products and services you need from our library via powerful searching tools