Histone lysine methylation imparts epigenetic information in chromatin biology, and the epigenetic signal transduction is mediated by multiple proteins containing methyllysine recognition domains. Some of the most well-known methyllysine recognition modules include Tudor, Chromo, MBT (malignant brain tumor), and PHD (plant homeodomain) domains. The Tudor domain is an important family of proteins because of its role as an adaptor and recruitment protein that promotes the formation of multiprotein complexes. Recognition of methylated histone tail lysine residues by Tudor domains plays important roles in epigenetic control of gene expression and DNA damage response.
Methyl lysine reader proteins are increasingly becoming explored as potential drug targets as increasing evidence is gained about their importance in the formation and progression of various diseases including cancers.
Creative BioMart offers access to the large collection of reader domain proteins, as well as a variety of reader domain assay services for screening, lead optimization or selectivity profiling. Creative BioMart currently provides high-throughput screening service based on AlphaScreen technology for the discovery of potential small-molecule inhibitors disrupting the interaction of histone with the Tudor domain protein.
Tudor Domain Screening Assay at Creative BioMart
We use a histidine-tagged Tudor domain and biotinylated histone peptides, which are captured by nickel chelate and streptavidin-coated AlphaScreen beads, respectively. The detection of the chemiluminescent readout depends on the binding of the protein and its cognate ligand. Typically, the donor bead captures a ligand while an acceptor bead captures the binding partner. The interaction of the protein and the ligand results in chemical energy transfer of acceptor and donor beads, ultimately producing a luminescent signal. Lack of binding does not allow the acceptor and donor beads to be sufficiently close and the singlet oxygen decays without producing light.
Advantages of Choosing Creative BioMart
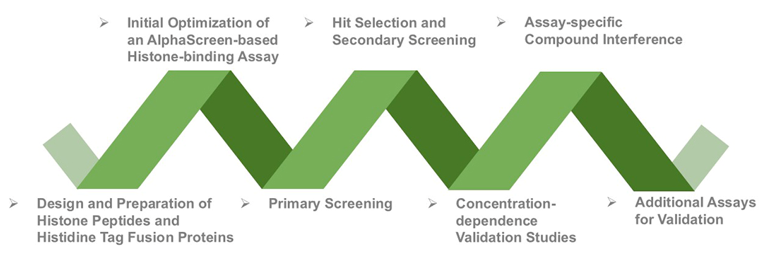
Figure 1. The Procedure of Tudor Domain Screening Assay
If you are interested in drug discovery associated with methyllysine recognition domains, please feel free to contact us for more information and a detailed quote.
References
1. Milosevich N.; et al. Structural aspects of small-molecule inhibition of methyllysine reader proteins. Future Medicinal Chemistry. 2016, 8(13): 1681-1702.
2. Wagner T.; et al. Identification of a small-molecule ligand of the epigenetic reader protein Spindlin1 via a versatile screening platform. Nucleic Acids Research. 2016: e88.
USA
Enter your email here to subscribe.
Follow us on

Easy access to products and services you need from our library via powerful searching tools