Cytoskeletal Filaments and Associated Proteins
About Cytoskeletal Filaments and Associated Proteins
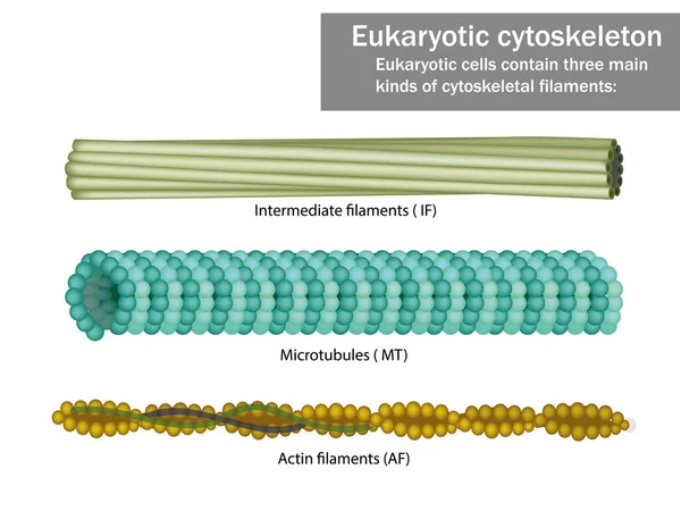
Cytoskeletal filaments are dynamic structures found in the cytoplasm of eukaryotic cells that play a crucial role in maintaining cell shape, providing mechanical support, and facilitating cell movement. There are three main types of cytoskeletal filaments: microfilaments, intermediate filaments, and microtubules.
1. Microfilaments, also known as actin filaments, are the thinnest cytoskeletal filaments, measuring about 7 nm in diameter. They are composed of actin proteins and are involved in various cellular processes, including cell contraction, cell division, and cell motility. Microfilaments can form a network underneath the plasma membrane, providing structural support and influencing cell shape.
2. Intermediate Filaments are intermediate-sized filaments, measuring about 10 nm in diameter. They are composed of different types of proteins such as keratins, vimentin, and neurofilament proteins. Intermediate filaments provide mechanical strength to cells and tissues, particularly in situations where cells are subject to mechanical stress. They are abundant in epithelial cells, where they help maintain tissue integrity.
3. Microtubules are the thickest cytoskeletal filaments, measuring about 25 nm in diameter. They are composed of tubulin proteins and play a vital role in cell division, cell shape maintenance, intracellular transport, and the organization of organelles. Microtubules can assemble and disassemble dynamically, providing a platform for the movement of various cellular components, such as vesicles and organelles.
In addition to the cytoskeletal filaments themselves, a multitude of associated proteins regulate their assembly, disassembly, and interactions with cellular structures. For example, actin-binding proteins modulate the polymerization and depolymerization of microfilaments, influencing their function and organization. Similarly, microtubule-associated proteins (MAPs) regulate the stability and dynamics of microtubules, as well as their interactions with other cellular components.
Overall, cytoskeletal filaments and associated proteins play crucial roles in maintaining cell shape, providing structural support, and facilitating various cellular processes.
The Significance of Studying Cytoskeletal Filaments and Associated Proteins
Cell Structure and Mechanical Support: Cytoskeletal filaments, including microtubules, actin filaments, and intermediate filaments, provide structural support to cells and contribute to their mechanical properties. Understanding the organization, dynamics, and interactions of cytoskeletal filaments helps elucidate how cells maintain their shape, withstand mechanical forces, and respond to external stimuli.
Cell Division and Motility: Cytoskeletal filaments play crucial roles in cell division and motility. Microtubules form the mitotic spindle, ensuring accurate chromosome segregation during cell division. Actin filaments drive cellular processes such as cytokinesis, cell migration, and intracellular transport. Intermediate filaments contribute to the mechanical integrity of cells and are involved in processes like cell adhesion and tissue integrity.
Intracellular Transport: Cytoskeletal filaments, along with associated motor proteins, facilitate intracellular transport of organelles, vesicles, and other cellular components. Microtubules serve as tracks for the movement of motor proteins like dynein and kinesin, enabling cargo transport to specific cellular destinations. Actin filaments also participate in vesicle trafficking and localized protein targeting.
Cell Signaling and Communication: Cytoskeletal filaments and associated proteins play a role in cell signaling and communication. They serve as platforms for signaling molecules, receptors, and scaffolding proteins, aiding in the transmission of signals between cells and within cellular compartments. Cytoskeletal dynamics also influence cellular responses to extracellular cues and modulate signaling pathways.
Development and Morphogenesis: The proper functioning and regulation of cytoskeletal filaments are crucial during embryonic development and tissue morphogenesis. Cytoskeletal dynamics drives processes such as cell migration, tissue patterning, and organogenesis. Disruptions in cytoskeletal organization can lead to developmental defects and diseases.
Disease Mechanisms: Malfunctions in cytoskeletal filaments and associated proteins are implicated in various diseases. For example, abnormalities in microtubules and associated proteins are linked to neurodegenerative disorders like Alzheimer's disease and Parkinson's disease. Mutations in actin and intermediate filament proteins are associated with muscular dystrophies and other genetic disorders. Studying these cytoskeletal components helps in understanding disease mechanisms and developing potential therapeutic interventions.
Biotechnology and Biomaterials: Cytoskeletal filaments and associated proteins have applications in biotechnology and biomaterials research. They can be utilized in the design of bioengineered tissues, scaffolds, and biomaterials to mimic or guide cellular behavior and tissue regeneration. Cytoskeletal components can also be employed as targets for drug development and screening assays.
The study of cytoskeletal filaments and associated proteins provides insights into fundamental cellular processes, disease mechanisms, and potential applications in various fields. It contributes to our understanding of cell biology, development, tissue engineering, and human health.
Available Resources for Cytoskeletal Filaments and Associated Proteins
Creative BioMart offers a wide range of products and custom services to support research with cytoskeletal filaments and related proteins. Our products cover recombinant proteins, cell and tissue lysates, protein pre-coupled beads, antibodies, and others, aiming to elucidate the mechanisms that control these important protein signaling pathways.
We also provide comprehensive resource support, including involved pathways, protein functions, interacting proteins, related research areas, related articles, and other items of cytoskeletal filaments and associated proteins, aiming to enhance the understanding and exploration of the functions and regulatory mechanisms of these important molecules.
We are dedicated to providing you with high-quality research tools and services to help you achieve successful scientific outcomes. If you have any further questions or require custom services, please feel free to contact us at any time.


