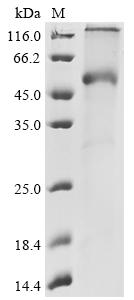
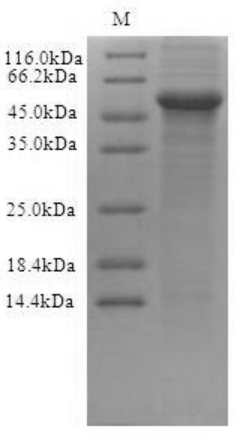
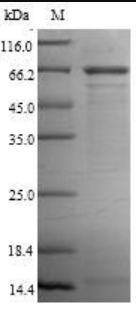
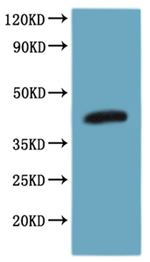
Transmembrane Proteins
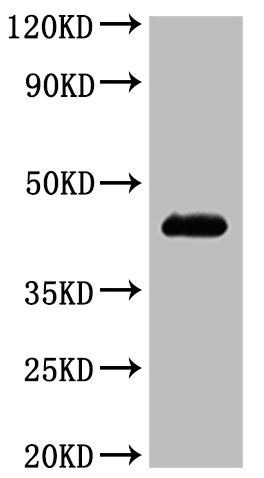
🧪 KCNE2-240H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-123aa
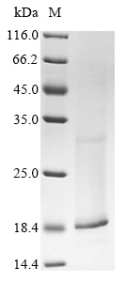
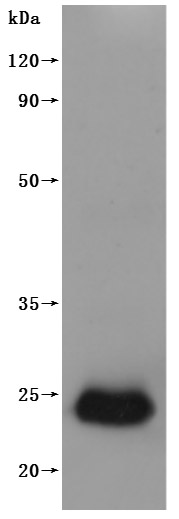
🧪 PVRIG-36H
Source: E.coli
Species: Human
Tag: His&SUMO
Conjugation:
Protein Length: 1-326aa
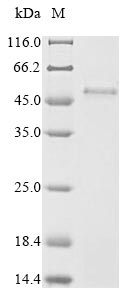
🧪 LMP1-01E
Source: Mammalian cells
Species: Epstein-Barr virus(HHV4)
Tag: His
Conjugation:
Protein Length: 1-386aa

🧪 GPRC5D-4280H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Met1-Val345
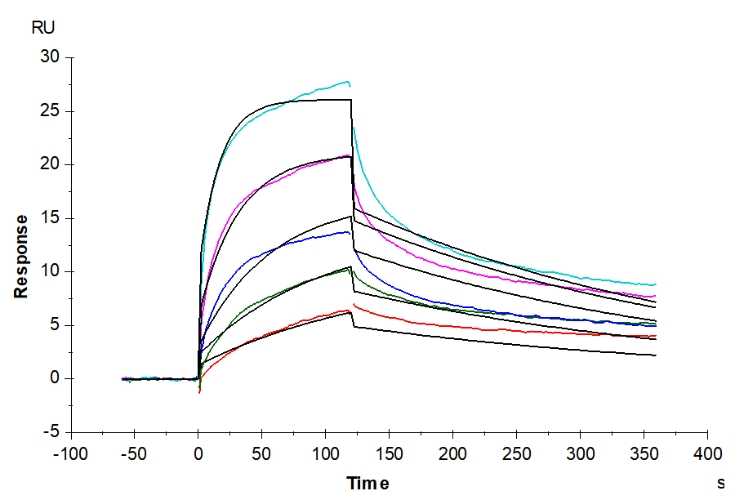
🧪 ADORA2A-0978H
Source: HEK293
Species: Human
Tag: Non
Conjugation:
Protein Length: Met 1 - Ser 412

🧪 PROM1-712H
Source: HEK293
Species: Human
Tag: Non
Conjugation:
Protein Length: Gly 20 - His 865

🧪 CXCR5-912H
Source: HEK293
Species: Human
Tag: Non
Conjugation:
Protein Length: Asn 2 - Phe 372

🧪 GPR56-523H
Source: HEK293
Species: Human
Tag: Non
Conjugation:
Protein Length: Arg 26 - Ile 693

Background
Overview
Transmembrane proteins are a large group of proteins in the cell membrane, which are those that have at least one region that spans the entire cell membrane. They are usually composed of hydrophobic amino acids in order to be able to interact with the hydrophobic tails in the phospholipid bilayer. The functions of transmembrane proteins are diverse and can be roughly divided into the following categories: receptor function, channel and pore function, transport function, enzyme activity, adhesion function, structural support, etc.
Classification
Transmembrane proteins are classified mainly based on how and where they interact with the cell membrane. It can be divided into the following categories:
Peripheral membrane proteins: These proteins adhere to the surface of the cell membrane by interacting with membrane lipid heads or integrated membrane proteins via non-covalent bonds. They do not penetrate deep into the lipid bilayer, so they are relatively easy to separate from the membrane.
Lipid-anchored membrane proteins: These proteins bind to membrane lipids via covalent bonds and are usually anchored to the membrane by glycophosphatidylinositol (GPI). They also do not penetrate deep into the lipid bilayer, but unlike peripheral membrane proteins, they are connected to the membrane lipid by covalent bonds.
Integrated membrane proteins: Also known as transmembrane proteins, they penetrate deep into the lipid bilayer, and some even span the entire lipid bilayer. Integrated membrane proteins can be divided into single transmembrane proteins, multiple transmembrane proteins and G protein-coupled receptors (GPCRS). These proteins play an important role in cell signaling and substance transport.
Ion channel proteins: These transmembrane proteins form channels that allow specific ions to pass through, such as sodium channels, potassium channels, and calcium channels.
G protein-coupled receptors (GPCRS): This is an important class of signal transduction proteins, including α-adrenergic receptors and β-adrenergic receptors, which play a role in the cell's response to external signaling molecules.
Transmembrane transporters: These proteins are responsible for transporting specific substances across cell membranes, such as ABC transporters and glucose transporters.
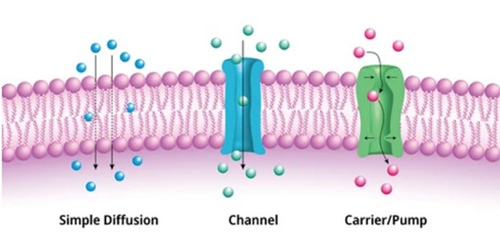
Challenges
The biggest challenges in the study of transmembrane proteins are the complexity of their structures and the difficulty in obtaining biologically active full-length proteins in vitro. These challenges are mainly reflected in the following aspects:
Difficult to express: transmembrane proteins are generally less expressed in host cells, possibly due to their complex structure and dependence on the cell membrane environment. This low level of expression makes it difficult to study, especially when large amounts of purified protein are required for experiments.
Poor stability: transmembrane proteins often have poor stability after leaving their natural cell membrane environment, and are prone to denaturation or aggregation, which affects their functional studies and structural analysis in vitro.
Complex structure: The structure of multiple transmembrane proteins is more complex, which leads to epitope accessibility problems and changes in the protein's dynamic conformation, which are unique obstacles to overcome in antibody drug development.
Solutions
In order to solve the difficult problem of transmembrane protein expression, the following strategies can be adopted:
Selection of a suitable host system: Since the hydrophobic region of transmembrane proteins is prone to inclusion bodies in prokaryotic cells, and prokaryotic cells have difficulty processing complex eukaryotic signaling peptides and post-translational modifications, choosing a eukaryotic expression system may be more conducive to the correct folding and expression of transmembrane proteins.
Optimization of expression conditions: The soluble expression level and function of transmembrane proteins can be improved by adjusting temperature, concentration of inducer, expression vector and host strain.
Use of solubilization labels: The introduction of solubilization labels, such as small ubiquitin associated modifier (SUMO) or maltose binding protein (MBP), can help increase the solubility of the target protein, thereby increasing the production of functional proteins.
Sequence optimization: Small adjustments to nucleotide or amino acid sequences can significantly affect the expression and folding of membrane proteins, which requires optimizing the sequence experimentally to improve expression levels.
Applications
- Vaccine development: Transmembrane proteins can be used to generate virus-like particles (VLPs) that highly mimic the structure of natural viruses, are not infectious and replicative, but can effectively induce an immune response and produce strong immune protection.
- Drug delivery: Because transmembrane proteins are able to embed in cell membranes, they can be used to develop novel drug delivery systems to more efficiently deliver drugs to specific cells or tissues.
- Biotherapy: as targets for drug research and development, transmembrane proteins are of great significance for the treatment of various diseases, especially major diseases such as cancer. The application of recombinant technology facilitates the development of antibodies and drugs targeting these proteins, thereby advancing the advancement of biotherapeutics.
- Bioenergy and biomaterials: Some transmembrane proteins are able to catalyze chemical reactions in the energy conversion process for bioenergy production. In addition, some transmembrane proteins can be used to make biological materials, such as biopolymers.
Case Study
Case Study 1: Active Recombinant Dog MS4A1 Full Length Transmembrane protein
ancer immunotherapies hold much promise, but their potential in veterinary settings has not yet been fully appreciated. Canine lymphomas are among the most common tumors of dogs and bear remarkable similarity to human disease. In this study, the researchers examined the combination of CD47 blockade with anti-CD20 passive immunotherapy for canine lymphoma. The CD47/SIRPα axis is an immune checkpoint that regulates macrophage activation. As robust synergy between CD47 blockade and tumor-specific antibodies has been demonstrated for human cancer, they evaluated the combination of CD47 blockade with 1E4-cIgGB, a canine-specific antibody to CD20. 1E4-cIgGB could elicit a therapeutic response against canine lymphoma in vivo as a single agent. However, augmented responses were observed when combined with CD47-blocking therapies, resulting in synergy in vitro and in vivo and eliciting cures in 100% of mice bearing canine lymphoma.
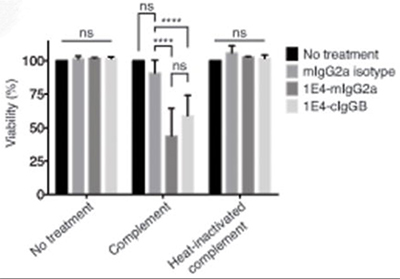
(Kipp Weiskopf, 2016)
Fig1. Both 1E4 and 1E4-cIgGB induced significant complement-mediated lysis of the target cells. Complement-dependent cytotoxicity assay using CLBL-1 cells cultured with 10 μg/ml mIgG2a, 1E4-mIgG2a, or 1E4-cIgGB in the presence of rabbit complement or heat-inactivated rabbit complement for 3 hours. Viability was normalized to the number of cells in the complement only condition using the MTS assay.
Case Study 2: Active Recombinant Human CCR4 Full Length Transmembrane protein
Studies indicate that the chemokine receptor is responsible for poor prognosis of hepatocellular carcinoma (HCC) patients. In this study, the researchers initially demonstrated that CCR4 is overexpressed in HCC specimens, and its elevation in HCC tissues positively correlates with tumor capsule breakthrough and vascular invasion. Although overexpression of CCR4 failed to influent proliferation of HCC cells in vitro apparently, the prominent acceleration on HCC tumor growth in vivo was remarkable.
The underlying mechanism may be involved in neovascularization. Interestingly, different from effect on proliferation, CCR4 overexpression could trigger HCC metastasis both in vitro and in vivo also induced HCC cell epithelial-mesenchymal transition (EMT) as well. Then we identified matrix metalloproteinase 2 (MMP2) as a direct target of CCR4 which plays an important role in CCR4-mediated HCC cell invasion, which was up-regulated by ERK/AKT signaling. Positive correlation between CCR4 and MMP2 expression was also observed in HCC tissues.
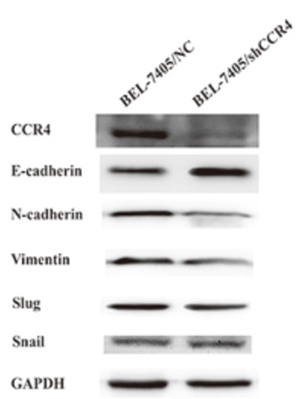
(Xi Cheng, 2017)
Fig2. CCR4 induces Epithelial-mesenchymal transition (EMT) in HCC cells. Western blot analyses showed that knockdown CCR4 in BEL-7405 cells could significantly decrease the expression of E-cadherin and increase the expression of N-cadherin and Vimentin.
Case Study 3: Active Recombinant Human CCR8 Full Length Transmembrane protein
Chemokines and their receptors could play key roles in the recruitment of T cells to the asthmatic lung. CCR8 is preferentially expressed on T-helper type 2 cells, and is thought to play a role in the pathogenesis of human asthma. This study aims to determine the expression of CCR8 on T cells in blood, bronchoalveolar lavage (BAL) and bronchial mucosa from asthmatics and normal subjects.
CCR8 expression in blood and BAL from asthma and normal subjects was studied using flow cytometry. CCR8 expression on IFN-gamma+ and IL-4+/IL-13+ blood and BAL T cells was studied following stimulation with Phorbol-Myristate-Acetate and Calcium Ionophore. Paraffin-embedded bronchial biopsies were used to study CCR8 in bronchial epithelium.
There was an approximately sixfold enrichment of CCR8 on IL-4+/IL-13+ cells compared with IFN-gamma+ T cells (P<0.001) in both asthmatic and normal subjects in both blood and BAL. Significantly more BAL T cells expressed CCR8 in asthmatic (8.6+/-0.8%) compared with normal subjects (3.9+/-0.7%) (P<0.01). In paired blood-BAL samples from asthmatics, significantly more CCR8+CD3+ T cells were present in BAL (9.0+/-0.9%) than in blood (5.6+/-0.9%; P<0.05).
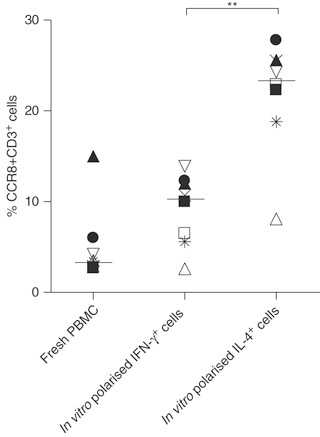
(K Mutalithas, 2010)
Fig3. T cell CCR8 expression among fresh peripheral blood mononuclear cells (PBMC), in vitro polarized IFN-γ+ and IL-4+ cells. Graph shows data from eight different experiments using PBMC from eight different asthmatic subjects. Bars represent median percentages. (**P<0.01 using the Mann–Whitney test).
Case Study 4: Active Recombinant Human CLDN6 Full Length Transmembrane protein
Recent studies have revealed that aberrant expression of tight junction (TJ) proteins is a hallmark of various solid tumors and it is recognized as a useful therapeutic target. Claudin-6 (CLDN6), a member of the family of TJ transmembrane proteins, is an ideal therapeutic target because it is not expressed in human adult normal tissues. In this study, the researchers found that CLDN6 is highly expressed in uterine cervical adenocarcinoma (ADC) and that high CLDN6 expression was correlated with lymph node metastasis and lymphovascular infiltration and was an independent prognostic factor. Shotgun proteome analysis revealed that cell-cell adhesion-related proteins and drug metabolism-associated proteins (aldo-keto reductase [AKR] family proteins) were significantly increased in CLDN6-overexpressing cells. Furthermore, overexpression of CLDN6 enhanced cell-cell adhesion properties and attenuated sensitivity to anticancer drugs including doxorubicin, daunorubicin, and cisplatin.

(Yui Ito, 2022)
Fig4. Overexpression of claudin‐6 (CLDN6) promotes the malignant transformation of cervical adenocarcinoma (ADC) cells. Expression of CLDN6 was undetectable in cervical ADC cell lines. N.D., not detected.
Advantages
- Wide Coverage: More than 500 transmembrane proteins from over 20 different species and 3 different sources.
- High Quality: Our transmembrane proteins have been tested with different methods to ensure intergrity and high purity.
- Experienced: We have professional research team with experience of many years in the field of molecular and cell biology.
- Fast turnaround: Under the premise of your protein expression and purification, as little as 4-6 weeks.
- Competitive pricing: We will deliver products according to your needs, and at a reasonable and satisfactory price.
FAQ
-
Q: How can the activity of recombinant protein be detected?
A: The bioactivity is measured by its binding ability in a functional ELISA and/or other activity assays.
-
Q: How pure is the recombinant protein?
A: The poreins have lower endotoxin level, less than 0.1 EU per μg protein by LAL assay.
Resources
References
- Weiskopf, K.; et al. Eradication of Canine Diffuse Large B-Cell Lymphoma in a Murine Xenograft Model with CD47 Blockade and Anti-CD20. Cancer Immunol Res. 2016;4(12):1072-1087.
- Cheng, X.; et al. Up-regulation of chemokine receptor CCR4 is associated with Human Hepatocellular Carcinoma malignant behavior. Sci Rep. 2017;7(1):12362.
- Mutalithas, K.; et al. Expression of CCR8 is increased in asthma. Clin Exp Allergy. 2010;40(8):1175-1185.
- Ito, Y.; et al. Aberrant expression of claudin-6 contributes to malignant potentials and drug resistance of cervical adenocarcinoma. Cancer Sci. 2022;113(4):1519-1530.