Early Mesodermal Lineage Markers
Related Symbol Search List
- BMP-2
- BMP4
- CDH12
- CFC1
- EOMES
- FABP4
- FGF5
- GDF1
- GDF3
- GSC2
- HAND1
- Activin A
- INHBB
- INHBC
- MIXL1
- NCLN
- NODAL
- SNAI1
- SNAI2
- TBX6
- Tgfb1
- TGFB2
- WNT3A
- WNT8A
Immunology Background
Background
The Building Blocks of Life
Imagine trying to construct a skyscraper without knowing where the foundation begins and ends. Just as architects need blueprints and markers to guide their work, biologists rely on specific markers to understand how our bodies develop from a single cell into a complex organism. One of the most fascinating and crucial aspects of this process involves the early mesodermal lineage markers, the signals that guide the formation of our muscles, bones, and blood vessels.
In the earliest stages of embryonic development, our cells are undifferentiated, meaning they have the potential to become any cell type in the body. As development progresses, these cells begin to specialize, guided by a series of signals and markers. The mesoderm is one of the three primary germ layers in the embryo, along with the ectoderm and endoderm. Cells of the mesodermal lineage are destined to form the vascular and lymphatic systems, including hemangioblasts (Hemangioblast Markers) and multipotent mesenchymal stem cells capable of differentiating into multiple specified cell types.
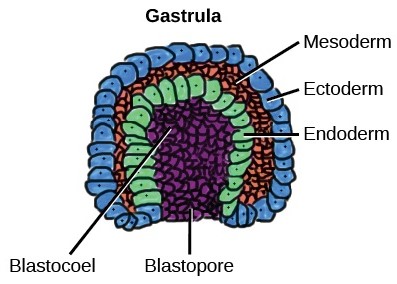 Fig. 1: The mesoderm is the middle layer of the three germ layers that develops during gastrulation in the very early development of the embryo of most animals. The outer layer is the ectoderm, and the inner layer is the endoderm (Wikipedia).
Fig. 1: The mesoderm is the middle layer of the three germ layers that develops during gastrulation in the very early development of the embryo of most animals. The outer layer is the ectoderm, and the inner layer is the endoderm (Wikipedia).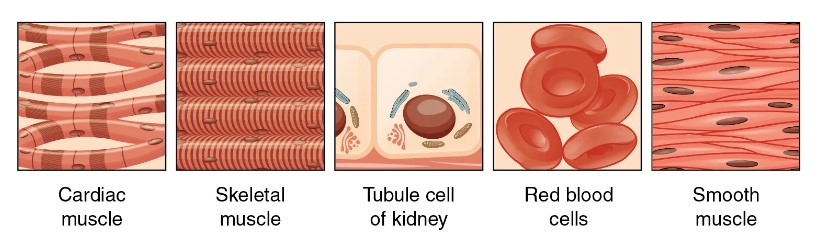 Fig. 2: Some of the tissues derived from mesoderm (Wikipedia).
Fig. 2: Some of the tissues derived from mesoderm (Wikipedia).Early mesodermal lineage markers are proteins and genes that indicate that a cell is beginning to differentiate into mesodermal tissue. These markers are critical for scientists to understand and track because they provide insight into the very basis of human development and can lead to advances in regenerative medicine and cancer research.
Key Early Mesodermal Markers
Some of the primary markers used to identify early mesodermal cells include:
- Snail (SNAI1, SNAI2): A zinc-finger transcription factor critical for the regulation of embryonic development. It plays a significant role in the process of mesoderm formation. Snail is involved in the epithelial-to-mesenchymal transition (EMT), a critical step in which cells lose their epithelial characteristics and gain migratory properties, allowing them to move to new locations and differentiate into different mesodermal tissues.
- Eomesodermin (Eomes): Eomes is crucial for the development of the early mesoderm and the formation of the heart and blood vessels. It also plays a role in the differentiation of T cells, which are vital for the immune system.
- Goosecoid (GSC): This gene is involved in the organization of the body plan of the developing embryo, influencing the formation of the head and the anterior-posterior axis.
- T-box transcription factor 6 (TBX6): TBX6 is a member of the T-box family of transcription factors, which are characterized by a conserved DNA-binding domain known as the T-box. TBX6 is a key early mesoderm marker critical for the development of the paraxial mesoderm, which gives rise to structures such as the vertebrae and skeletal muscles.
- Mixl1: Another transcription factor, Mixl1 is involved in the formation of mesodermal and endodermal tissues. It helps guide the cells to form the correct structures during embryonic development.
- Heart and Neural Crest Derivatives Expressed 1 (HAND1): HAN1 is a key early mesoderm marker and a basic helix-loop-helix (bHLH) transcription factor. It is essential for proper mesoderm development and plays an important role in cardiac and extraembryonic tissue development. Mutations or disruptions in HAND1 expression can lead to congenital heart defects and other developmental abnormalities, highlighting its critical role in early mesodermal development and organogenesis.
Applications
Understanding early mesodermal lineage markers has several significant implications:
Regenerative Medicine
By comprehending how these markers work, scientists can better direct stem cells to differentiate into specific cell types needed for tissue repair and regeneration. This knowledge could lead to breakthroughs in treating conditions like heart disease, osteoporosis, and muscle degeneration.
Cancer Research
Many cancers involve the abnormal differentiation of cells. By studying mesodermal markers, researchers can gain insight into how certain cancers develop and potentially discover new targets for therapy.
Developmental Biology
These markers help scientists understand the basic processes of human development. This knowledge can lead to a better understanding of birth defects and developmental disorders.
Drug Development
Identifying how cells transition from one state to another can help in the development of drugs that can modulate these processes. This could be particularly useful in developing treatments that promote tissue regeneration or inhibit cancer growth.
The study of early mesodermal lineage markers is a rapidly evolving field. With advances in genetic engineering and molecular biology techniques, researchers are uncovering new markers and their functions at an unprecedented pace. These discoveries not only deepen our understanding of human biology but also pave the way for innovative treatments and therapies.
As we continue to explore these cellular blueprints, we move closer to unlocking the full potential of regenerative medicine and bringing new hope to patients with previously untreatable diseases. The journey from a single cell to a fully formed human is a complex and miraculous process, and early mesodermal lineage markers are key to understanding this incredible transformation.
Quality Guarantee
We Guarantee the Quality of Our Products!
High Purity
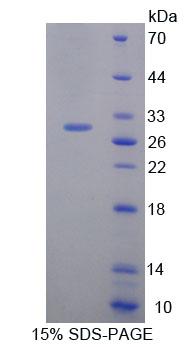 Fig. 3: SDS-PAGE of Hand1-7851M. The purity of the protein is greater than 90%.
Fig. 3: SDS-PAGE of Hand1-7851M. The purity of the protein is greater than 90%.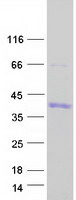 Fig. 4: SDS-PAGE of SNAI1-4718H. The purity of the protein is greater than 80%.
Fig. 4: SDS-PAGE of SNAI1-4718H. The purity of the protein is greater than 80%.Bioactivity
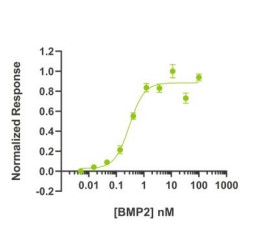 Fig. 5: BMP2 bioactivity is determined using a BMP2-responsive firefly luciferase reporter in stably transfected HEK293T cells. Cells are treated with a serial dilution of BMP2 for 6 hours. Firefly luciferase activity is measured and normalized to the control Renilla luciferase activity. EC50 = 7.81 ng/mL (0.3 nM).
Fig. 5: BMP2 bioactivity is determined using a BMP2-responsive firefly luciferase reporter in stably transfected HEK293T cells. Cells are treated with a serial dilution of BMP2 for 6 hours. Firefly luciferase activity is measured and normalized to the control Renilla luciferase activity. EC50 = 7.81 ng/mL (0.3 nM).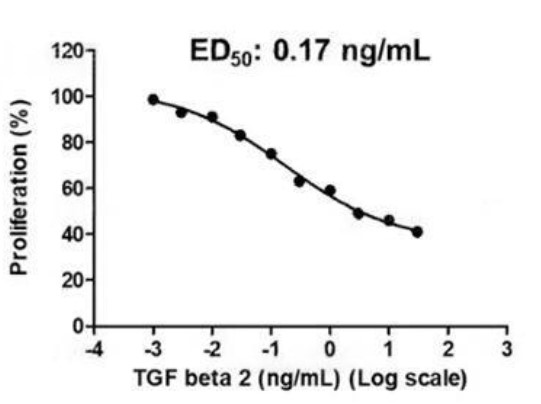 Fig. 6: Activity Data (TGFB2-287H).
Fig. 6: Activity Data (TGFB2-287H).Case Study
Case 1: Sun, S.; et al. The T-box transcription factor Brachyury promotes renal interstitial fibrosis by repressing E-cadherin expression. Cell Commun Signal. 2014 Nov 30;12:76.
The study showed that Brachyury was prominently induced in TGF-β1-treated human proximal tubule epithelial cells (HK-2) and that this induction was accompanied by changes characteristic of EMT. Meanwhile, cells overexpressing Brachyury downregulated the expression of the adhesion molecule E-cadherin, allowing them to undergo EMT. This process is mediated, at least in part, by the transcription factor Snail. In addition, overexpression of Brachyury may play a role in EMT associated with benign diseases such as renal fibrosis.
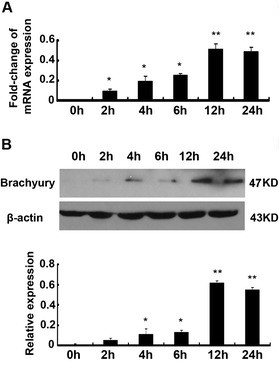 Fig. 7: Brachyury is induced rapidly during TGF-β1-mediated EMT. HK-2 cells treated with TGF-β1 showed increased mRNA levels of Brachyury by qRT-PCR. In addition, TGF-β1 treatment enriched Brachyury protein.
Fig. 7: Brachyury is induced rapidly during TGF-β1-mediated EMT. HK-2 cells treated with TGF-β1 showed increased mRNA levels of Brachyury by qRT-PCR. In addition, TGF-β1 treatment enriched Brachyury protein.Compared to control cells transfected with a control plasmid, overexpression of Brachyury resulted in decreased staining of E-cadherin and plakoglobin, while the staining of vimentin increased significantly. Interestingly, overexpression of Brachyury inhibited the expression of epithelial cell markers E-cadherin and plakoglobin in tubule epithelial cells, while the levels of fibronectin, α-sliding muscle actin (α-SMA) and vimentin were increased. The results showed that brachyury can cause EMT of tubule cells in vitro.
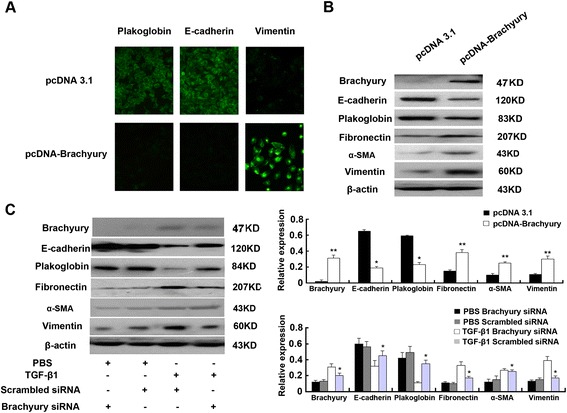 Fig. 8: Brachyury mediates TGF-β1-induced EMT in HK-2 cells.
Fig. 8: Brachyury mediates TGF-β1-induced EMT in HK-2 cells.Case 2: Liu, Z.; et al. Wnt ligands 3a and 5a regulate proliferation and migration in human fetal liver progenitor cells. Transl Gastroenterol Hepatol. 2021 Oct 25;6:56.
The canonical wingless/int-1 (Wnt) signal transduction pathway plays a key role in proliferation and migration of stem cells. This study a role for Wnt3a and Wnt5a ligands in regulating the proliferation and migration of hFLPC (since human fetal liver progenitor cells), which can differentiate into multiple liver cell types in vitro and in vivo and may be a suitable source for cell therapy and regeneration strategies. Using alamarBlue assay and transwell migration assay, the proliferation and migration of hFLPC to Wnt3a and Wnt5a were examined. The results showed that Wnt3a or Wnt5a significantly increased migration and proliferation independently in a dose-dependent manner, which was significantly inhibited by Wnt inhibitors.
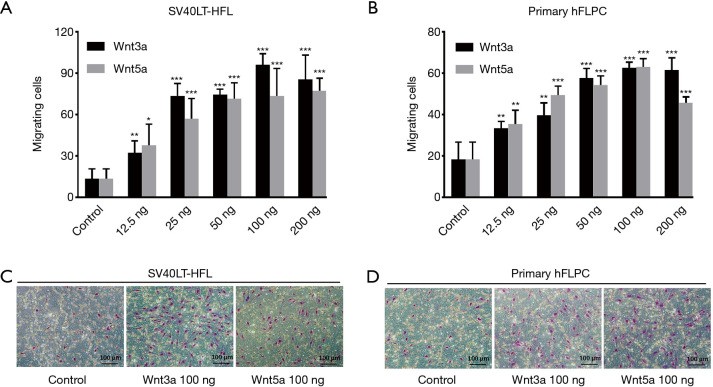 Fig. 9: Wnt ligands 3a and 5a induced migration of SV40LT-HFL cells and primary hFLPC. The representative microscopic pictures of transwell after Giemsa staining showing high number of migrated cells to Wnt3a and Wnt5a at 100 ng concentration compared to control for SV40LT-HFL cells (C) and primary hFLPC (D).
Fig. 9: Wnt ligands 3a and 5a induced migration of SV40LT-HFL cells and primary hFLPC. The representative microscopic pictures of transwell after Giemsa staining showing high number of migrated cells to Wnt3a and Wnt5a at 100 ng concentration compared to control for SV40LT-HFL cells (C) and primary hFLPC (D).Reference
- Evseenko, Denis, et al. "Mapping the First Stages of Mesoderm Commitment during Differentiation of Human Embryonic Stem Cells." Proceedings of the National Academy of Sciences of the United States of America, vol. 107, no. 31, Aug. 2010, pp. 13742–47. PubMed, https://doi.org/10.1073/pnas.1002077107.
- Liu, Zhiwen, et al. "Wnt Ligands 3a and 5a Regulate Proliferation and Migration in Human Fetal Liver Progenitor Cells." Translational Gastroenterology and Hepatology, vol. 6, Oct. 2021, pp. 56–56. DOI.org (Crossref), https://doi.org/10.21037/tgh.2020.01.12.
- Scialdone, Antonio, et al. "Resolving Early Mesoderm Diversification through Single-Cell Expression Profiling." Nature, vol. 535, no. 7611, July 2016, pp. 289–93. PubMed, https://doi.org/10.1038/nature18633.
- Stavish, Dylan, et al. "Generation and Trapping of a Mesoderm Biased State of Human Pluripotency." Nature Communications, vol. 11, no. 1, Oct. 2020, p. 4989. PubMed, https://doi.org/10.1038/s41467-020-18727-8.
- Sun, Shiren, et al. "The T-Box Transcription Factor Brachyury Promotes Renal Interstitial Fibrosis by Repressing E-Cadherin Expression." Cell Communication and Signaling: CCS, vol. 12, 2014. www.ncbi.nlm.nih.gov, https://doi.org/10.1186/s12964-014-0076-4.

