Adaptive Immunity
Creative BioMart Adaptive Immunity Product List
Immunology Background
Background
About Adaptive Immunity
Adaptive immunity, also known as acquired immunity or specific immunity, is a sophisticated defense mechanism found in vertebrates, including humans, that provides long-lasting protection against specific pathogens. It is characterized by its ability to recognize and mount targeted responses against a wide range of foreign substances, including bacteria, viruses, parasites, and toxins.
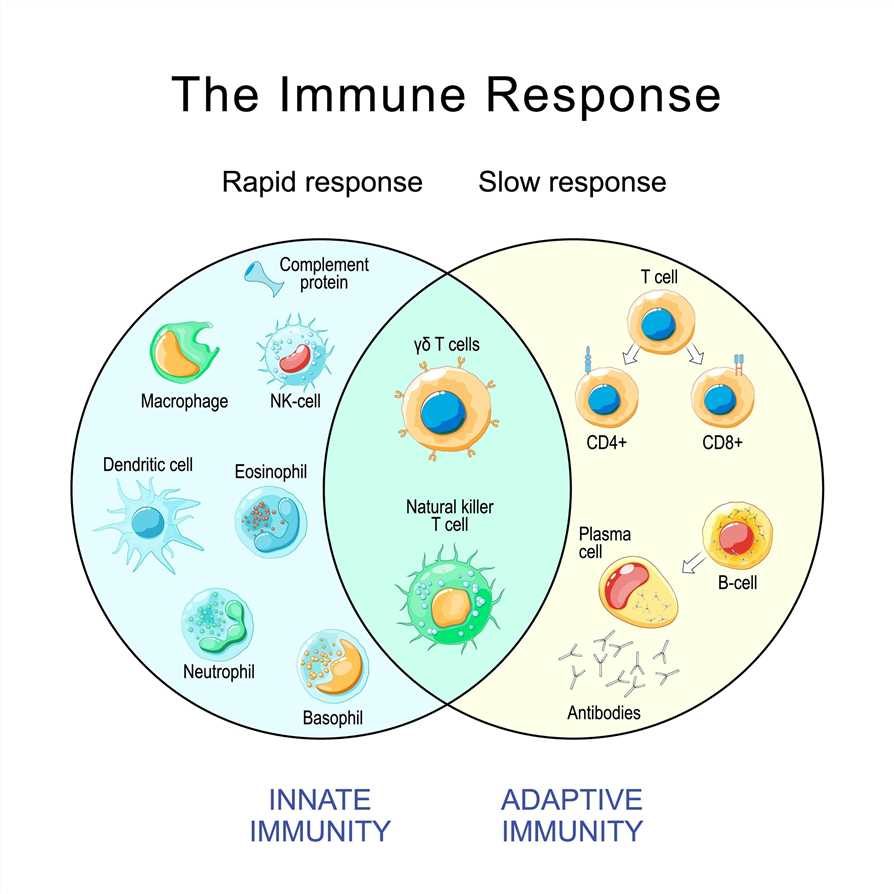
Characteristics and Functions of Adaptive Immunity
Adaptive immunity is distinguished from innate immunity, which provides immediate, non-specific defense mechanisms, such as barriers (e.g., skin and mucous membranes) and cells (e.g., natural killer cells and phagocytes). Adaptive immunity possesses several key characteristics and functions that make it a crucial component of the immune system. Here are the main characteristics and functions of adaptive immunity:
| Characteristics | Functions |
|---|---|
|
Adaptive immunity has a remarkable ability to recognize and respond to a vast array of antigens. B cells and T cells possess antigen-specific receptors that can recognize specific epitopes on pathogens. This specificity allows the immune system to mount targeted responses against particular pathogens, increasing the efficiency of the immune response. |
|
One of the key features of adaptive immunity is immunological memory. After an initial encounter with an antigen, memory B cells and memory T cells are generated. These memory cells "remember" the antigen, allowing for a rapid and robust immune response upon re-exposure to the same pathogen. Immunological memory is the basis for long-lasting protection provided by vaccines and is crucial in preventing reinfection. |
|
Adaptive immunity is capable of recognizing and responding to diverse pathogens, including viruses, bacteria, fungi, parasites, and even abnormal cells like cancer cells. This versatility enables the immune system to provide defense against a wide range of infectious agents and abnormal cell growth. |
|
Adaptive immunity has mechanisms in place to ensure self-tolerance, meaning it does not mount immune responses against the body's own cells and tissues. Central tolerance occurs during immune cell development in the thymus and bone marrow, where self-reactive lymphocytes are eliminated or rendered tolerant. Peripheral tolerance mechanisms further regulate immune responses in the periphery to prevent autoimmunity. |
|
Adaptive immunity plays a crucial role in regulating immune responses. Helper T cells, a subset of T cells, release cytokines that coordinate and modulate immune responses. They help in activating other immune cells, such as B cells and cytotoxic T cells, and regulate the intensity and duration of immune reactions, preventing excessive inflammation and autoimmune responses. |
|
Adaptive immunity participates in immunological surveillance, continuously monitoring the body for the presence of foreign invaders or abnormal cells. Through antigen recognition and targeted responses, adaptive immunity helps identify and eliminate pathogens, infected cells, and cancerous cells, contributing to overall immune defense. |
Two Major Branches of Adaptive Immunity
There are two main components of adaptive immunity: humoral immunity and cellular immunity (cell-mediated immunity). Here's a closer look at each branch:
| Branches | Detailed introduction |
|---|---|
| Cellular Immunity | Cellular immunity involves the activation of specialized immune cells, primarily T cells, to directly target and eliminate infected cells, abnormal cells, and pathogens. Key components and processes of cellular immunity include:
|
| Humoral Immunity | Humoral immunity involves the production and circulation of antibodies (immunoglobulins) in the body's fluids, such as blood and lymph. It is primarily mediated by B cells and is responsible for neutralizing pathogens, facilitating their clearance, and providing long-term immunity. Key components and processes of humoral immunity include:
|
Cellular immunity and humoral immunity work in tandem to provide a comprehensive and coordinated adaptive immune response. While cellular immunity primarily targets intracellular pathogens and abnormal cells, humoral immunity focuses on neutralizing extracellular pathogens and toxins. The balance and collaboration between these two branches contribute to the overall effectiveness of adaptive immunity in protecting the body against a wide range of infectious agents.
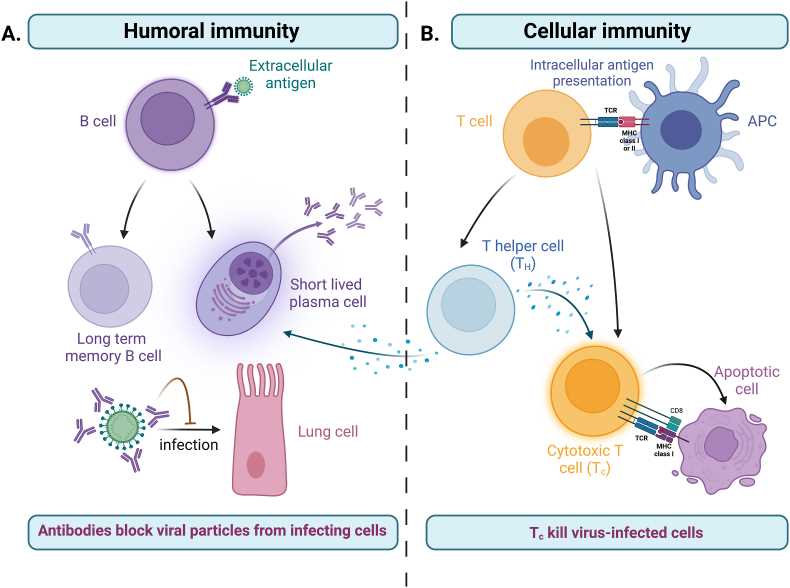 Fig.2 The two arms of the adaptive immune response. (Schwarz M, et al., 2022)
Fig.2 The two arms of the adaptive immune response. (Schwarz M, et al., 2022)Key Cells and Molecules in Adaptive Immunity
In adaptive immunity, several key cells and molecules play essential roles in orchestrating immune responses. Here are the key components involved:
- B Cells: B cells are lymphocytes that play a central role in humoral immunity. They are responsible for the production and secretion of antibodies. B cells express B cell receptors (BCRs) on their surface, which can recognize specific antigens. When activated by antigen binding, B cells differentiate into plasma cells that secrete large amounts of antibodies, providing a key defense against pathogens.
- T Cells: T cells are another type of lymphocyte involved in adaptive immunity. They are responsible for cell-mediated immunity and have different subsets with distinct functions. Helper T cells (CD4+ T cells) assist other immune cells by releasing cytokines and providing help for B cells and cytotoxic T cells. Cytotoxic T cells (CD8+ T cells) directly kill infected cells or tumor cells.
- Co-stimulatory and Co-inhibitory Molecules: Co-stimulatory and co-inhibitory molecules are crucial regulators of T cell activation and function. Co-stimulatory molecules, such as CD28 on T cells and CD80/86 on antigen-presenting cells (APCs), provide necessary signals for T cell activation and proliferation. Co-inhibitory molecules, such as CTLA-4 and PD-1, regulate the intensity and duration of the immune response, preventing excessive activation and autoimmune reactions.
- Major Histocompatibility Complex (MHC): The MHC, also known as the Human Leukocyte Antigen (HLA) system in humans, is a set of genes encoding cell surface proteins involved in antigen presentation. MHC molecules are divided into two classes: MHC class I and MHC class II. MHC class I molecules are expressed on the surface of most nucleated cells and present antigenic peptides to cytotoxic T cells. MHC class II molecules are primarily expressed on professional antigen-presenting cells (APCs) such as dendritic cells, macrophages, and B cells. They present antigenic peptides to helper T cells.
The interaction between T cells and APCs, facilitated by MHC molecules and co-stimulatory molecules, is essential for antigen recognition and initiation of an adaptive immune response. The activation and coordination of B cells and T cells, along with the involvement of co-stimulatory and co-inhibitory molecules, orchestrate a highly specific and regulated immune response against pathogens.
Understanding the functions and interactions of these key cells and molecules in adaptive immunity is crucial for developing therapeutic approaches, such as vaccines, immunotherapies, and targeted treatments for immune-related disorders.
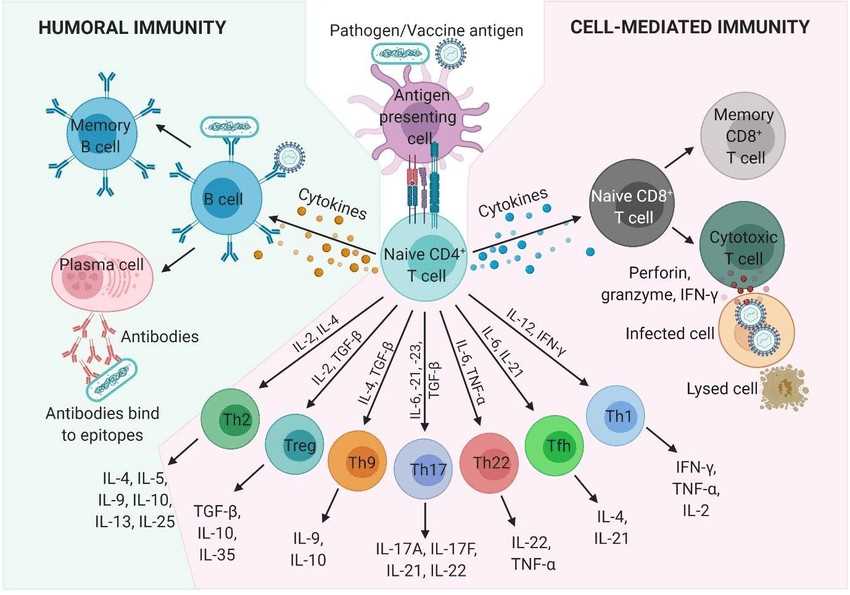 Fig.3 Humoral and cell-mediated adaptive immune responses and the cells and molecules involved. (Schijns V, et al., 2021)
Fig.3 Humoral and cell-mediated adaptive immune responses and the cells and molecules involved. (Schijns V, et al., 2021)Working Principle of Adaptive Immunity
Adaptive immunity is a complex and coordinated system that involves multiple steps and interactions to mount a targeted immune response against specific pathogens. Here is an overview of how adaptive immunity works:
- Antigen Recognition and Processing
The process begins when immune cells, such as dendritic cells, macrophages, or B cells, encounter foreign antigens on pathogens or abnormal cells. These antigens are specific molecular components or structures that are recognized as "non-self" by the immune system. The immune cells capture and process the antigens, breaking them down into smaller fragments. - Antigen Presentation
The processed antigen fragments are then displayed on the surface of the antigen-presenting cells (APCs) using specialized molecules called major histocompatibility complexes (MHCs). MHC class I molecules present antigens to cytotoxic T cells (CD8+ T cells), while MHC class II molecules present antigens to helper T cells (CD4+ T cells). - T Cell Activation
The presented antigens on APCs interact with the specific T cell receptors (TCRs) on T cells that possess complementary antigen specificity. This interaction, accompanied by co-stimulatory signals, triggers the activation of T cells. Cytotoxic T cells (CD8+ T cells) become activated when their TCRs recognize antigen-MHC class I complexes, while helper T cells (CD4+ T cells) become activated when their TCRs recognize antigen-MHC class II complexes. - Helper T Cell Differentiation
Activated helper T cells play a critical role in directing and coordinating the immune response. They differentiate into different subsets depending on the cytokines present in their environment. The main helper T cell subsets are Th1, Th2, Th17, and Tregs. Each subset produces specific cytokines that influence the type and magnitude of the immune response, activating other immune cells such as B cells, cytotoxic T cells, and macrophages. - B Cell Activation and Antibody Production
Activated helper T cells interact with B cells that have encountered the same antigen. This interaction, along with additional signals, stimulates B cell activation and differentiation. B cells differentiate into plasma cells, which are specialized antibody-producing cells. Plasma cells produce and secrete large quantities of antibodies (immunoglobulins) that specifically bind to the antigen, marking it for destruction or neutralization. - Antibody Functions
Antibodies play various roles in the immune response. They can directly neutralize pathogens, preventing their entry into host cells or blocking their harmful effects. Antibodies can also tag pathogens for destruction by other immune cells through a process called opsonization. Additionally, antibodies can activate the complement system, a cascade of proteins that leads to pathogen lysis or enhanced phagocytosis. - Memory Cell Formation
During the immune response, memory B cells and memory T cells are generated. These long-lived cells "remember" the specific antigen encountered, providing long-term immunity. If the same antigen is encountered again, memory cells can mount a faster and more robust response, leading to quicker clearance of the pathogen. - Immune Regulation
Adaptive immunity includes regulatory mechanisms to prevent excessive immune responses and maintain immune homeostasis. Regulatory T cells (Tregs) play a critical role in suppressing immune reactions and preventing autoimmune responses. They help regulate the intensity and duration of immune responses, ensuring a balanced immune system.
The process of adaptive immunity involves a dynamic interplay between different immune cells, cytokines, and signaling molecules. This coordinated response allows the immune system to specifically recognize and eliminate pathogens, prevent reinfection, and establish long-term immunity.
Future research directions and development trends in the field of adaptive immunity are diverse and continuously evolving as scientists strive to deepen their understanding and harness the potential of this critical component of the immune system. Here are some prominent areas of focus:
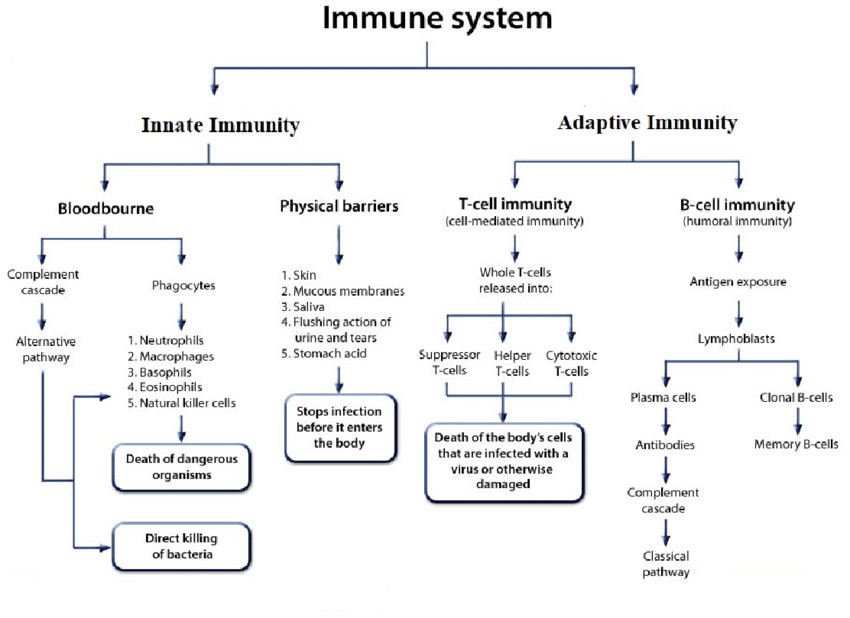 Fig.4 Flowchart of basic components of human Immune system. (Sujata Paul, et al., 2020)
Fig.4 Flowchart of basic components of human Immune system. (Sujata Paul, et al., 2020)Future Research Directions and Development Trends of Adaptive Immunity
- Immunotherapies and Vaccines: There is a growing interest in developing innovative immunotherapies and vaccines that leverage the power of adaptive immunity. Research is focused on designing novel vaccine platforms, improving vaccine delivery systems, and identifying new adjuvants to enhance immune responses. Additionally, advances in adoptive cell therapies, such as chimeric antigen receptor (CAR) T cell therapy, are being explored to harness the potential of T cell-mediated immunity for targeted cancer treatments.
- Immune Memory and Long-Term Protection: Understanding the mechanisms that underlie immune memory and long-term protection is a key research area. Scientists aim to elucidate the cellular and molecular processes involved in generating and maintaining immunological memory, as well as strategies to enhance memory responses. This knowledge can guide the development of more effective vaccines and immunotherapies and aid in the design of interventions against persistent infections and chronic diseases.
- Immune Regulation and Tolerance: Investigating the mechanisms of immune regulation and tolerance is crucial for preventing or treating autoimmune diseases and improving transplantation outcomes. Researchers are exploring the role of regulatory T cells (Tregs), immune checkpoints, and immune suppressive pathways to gain insights into immune tolerance and develop targeted therapeutic interventions.
- Systems Immunology and Big Data Analysis: The advancement of high-throughput technologies and computational tools has enabled the field of systems immunology. This interdisciplinary approach integrates large-scale data analysis, computational modeling, and experimental techniques to understand the complex interactions and dynamics of the immune system. Systems immunology can provide a more comprehensive understanding of adaptive immunity and aid in the identification of new therapeutic targets and biomarkers.
- Personalized Medicine and Precision Immunology: The concept of personalized medicine is gaining traction in the field of adaptive immunity. Researchers are investigating individual variations in immune responses and genetic factors that influence immune function. This knowledge can facilitate the development of personalized immunotherapies, vaccines, and diagnostics tailored to an individual's immune profile, leading to more targeted and effective treatments.
- Aging and Immunity: With an aging population, understanding the impact of age on adaptive immunity is crucial. Researchers are studying age-related changes in immune function, known as immunosenescence, to identify strategies for maintaining or restoring immune competence in older individuals. This research can inform the development of interventions to enhance vaccine responses and combat age-related diseases.
- Host-Microbiome Interactions: The role of the microbiome, the collection of microorganisms residing in and on the human body, in modulating adaptive immunity is an emerging area of investigation. Research is focused on understanding how the microbiome influences immune development, function, and responses to infections and autoimmune diseases. Manipulating the microbiome may offer new avenues for modulating adaptive immune responses.
These research directions and development trends aim to deepen our understanding of adaptive immunity, uncover new therapeutic approaches, and improve immune-based interventions. Continued advancements in technology, collaboration among researchers, and the translation of scientific discoveries into clinical applications hold promise for revolutionizing the field of adaptive immunity and its impact on human health.
Case Study
Case 1: Li C, Lee A, Grigoryan L, et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat Immunol. 2022;23(4):543-555.
The BNT162b2 mRNA vaccine developed by Pfizer and BioNTech is the first US Food and Drug Administration (FDA)-approved mRNA vaccine in history. The vaccine demonstrated 95% efficacy against symptomatic coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a phase-III trial. Several studies have analyzed immune responses to the BNT162b2 vaccine in humans and revealed insights about the nature of the antibody and T cell responses to vaccination.
The authors conducted an evaluation of immune responses to BNT162b2, a COVID-19 vaccine. They immunized C57BL/6 mice with BNT162b2 and observed the following results:
a) Mice were immunized with BNT162b2 on days 0 and 21 using intramuscular injection.
b) Anti-spike binding IgG responses, including both IgG1 and IgG2c, significantly increased 14 days after the initial immunization and remained elevated until day 21. The secondary immunization further boosted the response by more than tenfold.
c) Neutralizing antibody responses against the SARS-CoV-2 wild-type strain and various variants of concern, including alpha, beta, gamma, delta, and epsilon, were detected on day 21 and significantly increased after the secondary immunization. These findings were consistent with observations in humans.
d-f) In addition to serological responses, the researchers examined the responses of germinal center B cells (GC B cells), T follicular helper cells (TFH cells), and plasma cells in the draining lymph nodes (dLNs). BNT162b2 immunization induced a robust increase in GC B cells, TFH cells, and plasma cells, peaking on day 7 and declining by day 21.
Overall, the study demonstrated that BNT162b2 strongly stimulated humoral immune responses, including antibody production and the activation of specific immune cell populations.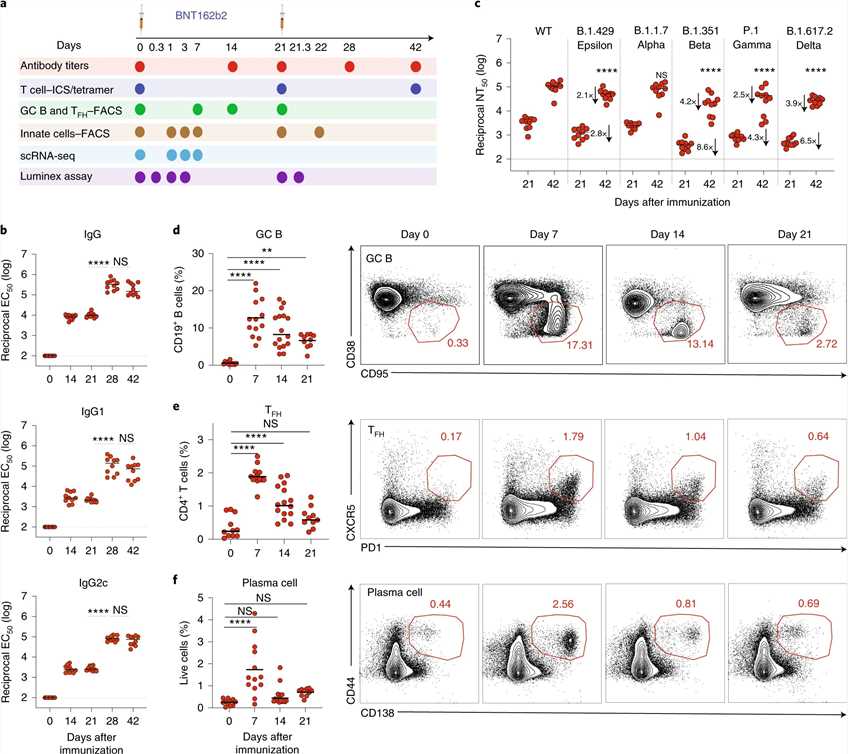 Fig.1 BNT162b2 vaccine induces robust germinal center B cell and TFH cell response in mice.
Fig.1 BNT162b2 vaccine induces robust germinal center B cell and TFH cell response in mice.References
- Schwarz M, Mzoughi S, Lozano-Ojalvo D, Tan AT, Bertoletti A, Guccione E. T Cell Immunity is Key to The Pandemic Endgame: How To Measure and Monitor It. Curr Res Immunol. 2022;3:215-221.
- Schijns V, Majhen D, van der Ley P, et al. Rational Vaccine Design in Times of Emerging Diseases: The Critical Choices of Immunological Correlates of Protection, Vaccine Antigen and Immunomodulation. Pharmaceutics. 2021;13(4):501.
- Paul, Sujata & Hmar, Elbethel Lalthavel & Sharma, Hemanta. (2020). Strengthening Immunity With Immunostimulants: A Review. Current Trends in Pharmaceutical Research. 7. 35-64.

