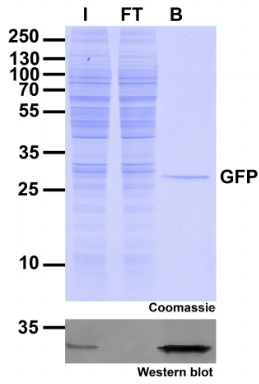
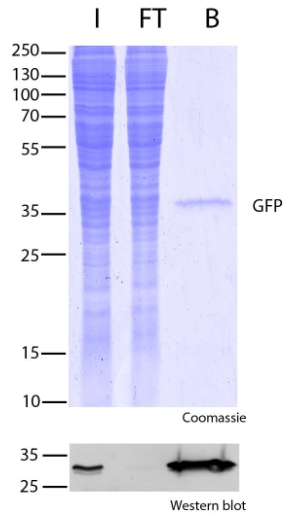
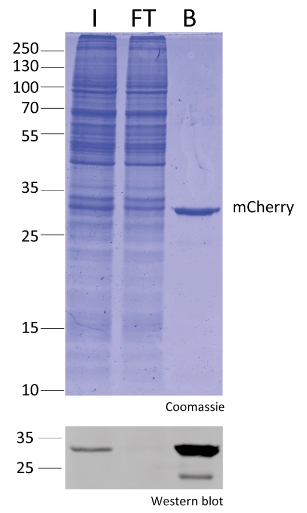
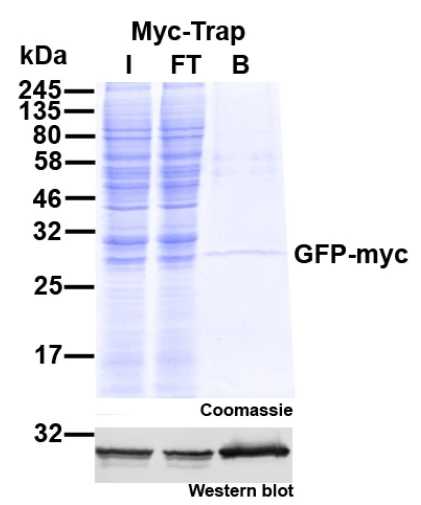
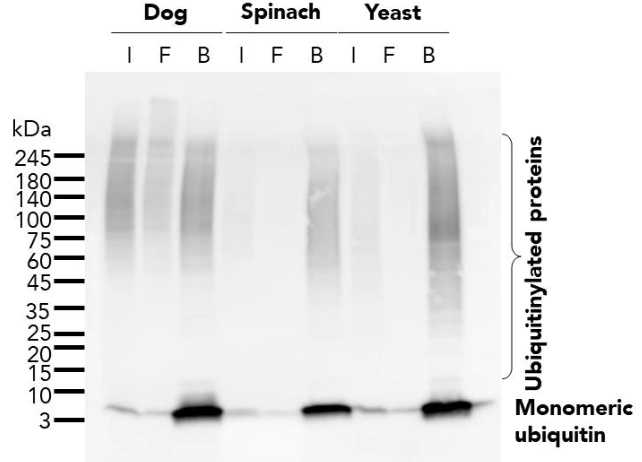
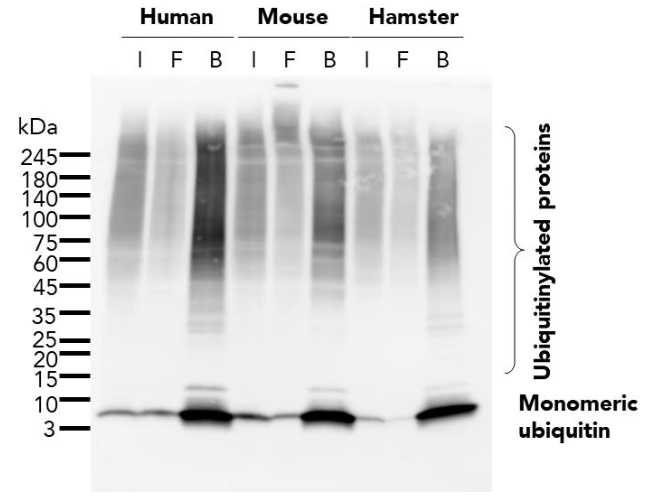
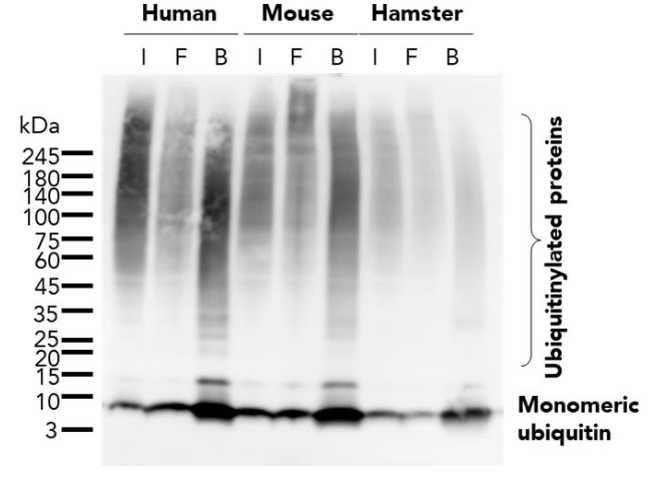
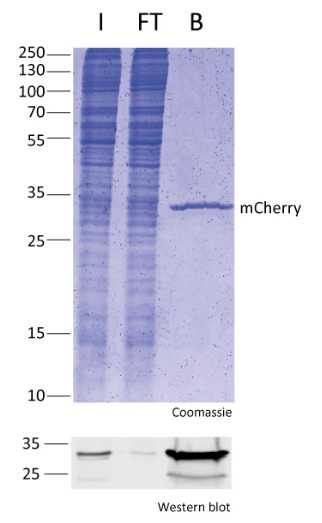
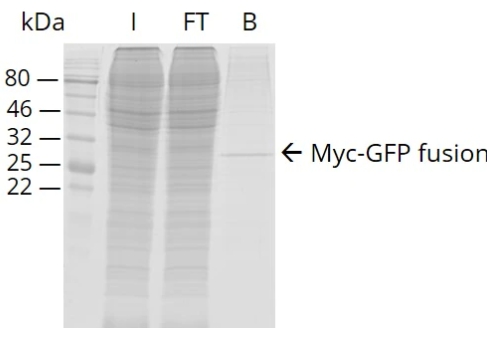
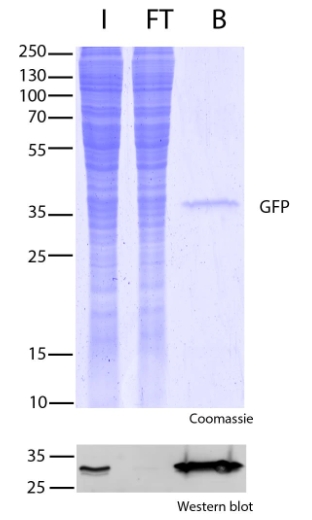
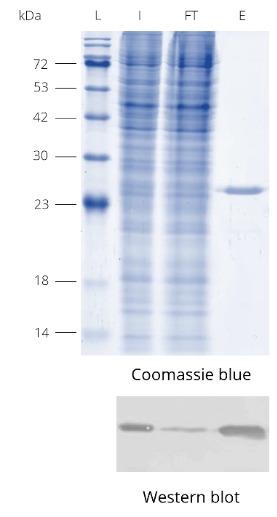
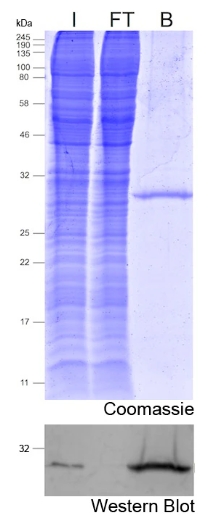
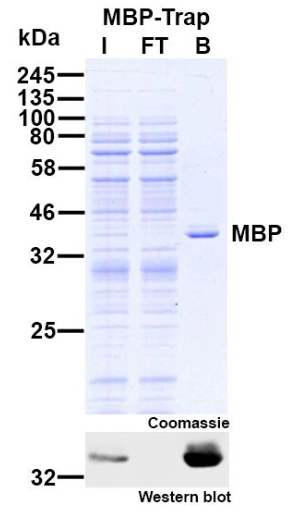
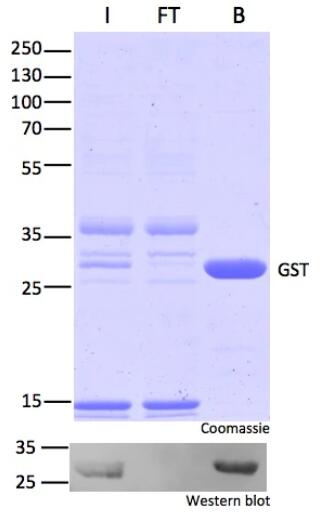
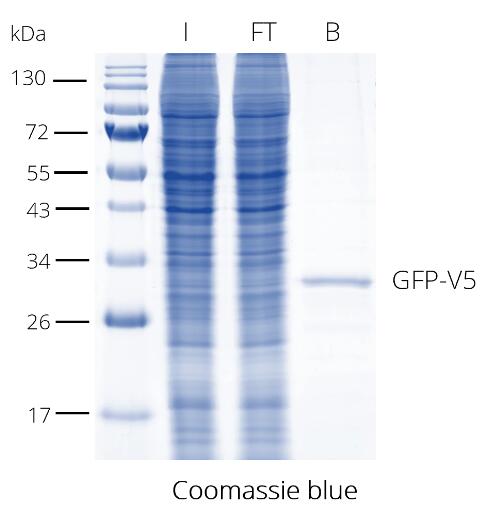
Protein Pre-coupled Magnetic Beads
SubCategories
Background
Overview
Protein precoupled magnetic beads are a biotechnology tool that uses the specific interaction between magnetic particles and proteins to isolate and enrich target proteins. It usually consists of magnetic particles and specific recognition proteins, such as antibodies.
The preparation process of protein precoupled magnetic beads generally includes the following steps: First, the magnetic particles and specific recognition proteins (such as antibodies) are chemically coupled to form a conjugate; Then, the conjugate reacts with the target protein to bind the target protein to the conjugate. Finally, the magnetic particles bound to the target protein are separated through the action of magnetic field, so as to achieve the enrichment and purification of the target protein.

Research Progress
The origins of protein-precoupled magnetic beads date back to the 1970s, when researchers began exploring ways to use magnetic particles to separate and enrich biomolecules. With the continuous development of technology, protein precoupled magnetic beads have gradually become an important biotechnology tool and have been widely used in life science research. In the development of protein precoupled magnetic beads, the following key events are worth mentioning:
1. In the early 1990s, Japanese scientist Shinya Yamanaka invented another technique called "genome editing," which is able to precisely modify a cell's genome. The emergence of this technology has further promoted the research and application of protein precoupled magnetic beads.
2. In 1995, American scientists Richard Scheller and Michael Smith invented a method called "protein affinity chromatography", which uses specific interactions between proteins and magnetic particles to enrich and purify target proteins. This method provides the basis for the application of protein precoupled magnetic beads.
3. After 2000, with the development of nanotechnology and bioengineering technology, the function and performance of protein pre-coupled magnetic beads have been greatly improved. At the same time, people also began to explore the application of protein precoupled magnetic beads in clinical medicine and other fields.
Advantages of Protein Precoupled Magnetic Beads
- High sensitivity and selectivity: Protein precoupled magnetic beads use the interaction between specific antibodies and magnetic particles to enrich and purify the target protein, so they have high sensitivity and selectivity.
- Easy to operate and fast: The operation of protein precoupled magnetic beads is relatively simple, usually only need to add the sample to the buffer containing magnetic particles, and the magnetic field can be separated. The whole process usually takes only a few minutes to tens of minutes.
- Good automation performance: protein precoupled magnetic beads can be used in combination with a variety of biological instruments (such as automatic pipettes, magnetic racks, etc.) to achieve automated operation, reducing the error and labor intensity of manual operation.
- Good repeatability: The preparation process of protein precoupled magnetic beads is relatively standardized and has good repeatability. This makes the results of experiments conducted by different experimentalists at different times and places comparable.
- Suitable for the separation of complex samples: Protein precoupled magnetic beads can effectively separate and enrich complex biological samples, such as cell lysate, serum, etc. This makes it have a wide application prospect in life science research.
- Can be used to analyze the interaction between biomolecules: Protein precoupled magnetic beads can be used to study the interaction between proteins, nucleic acids, small molecules and other biomolecules, thereby revealing the function and regulatory mechanism of biomolecules.

Separation Principle
The separation principle of protein precoupled magnetic beads is to use the specific interaction between magnetic particles and specific recognition proteins (such as antibodies) to achieve the enrichment and purification of target proteins. Specifically, protein precoupled magnetic beads are usually composed of magnetic particles (such as gold nanoparticles, magnetic ferric oxide particles, etc.) and specific recognition proteins (such as antibodies). When the target proteins are present in the sample, they bind to the antibodies that are coupled to the magnetic particles, forming a magnetic particle-antibody-target protein complex. The sample containing the complex is then placed in a magnetic field, which attracts the magnetic particles together due to their spontaneous magnetization in the same direction, thus achieving the separation of the target protein.
In addition to magnetic particles, protein precoupled magnetic beads can also contain other auxiliary molecules, such as elution buffers, surfactants, etc., to aid in the release and separation of target proteins. By changing the pH value and ionic strength of the buffer, the stability of the magnetic particle-antibody-target protein complex can be adjusted, so as to control the release and separation effect of the target protein.
Applications
Protein precoupled magnetic beads have a wide range of applications, including but not limited to:
- Biological analysis : Protein precoupled magnetic beads can be used to separate, purify, enrich specific proteins in biological samples, such as antibodies in serum, proteins in cells, etc.
- Bioengineering : Protein precoupled magnetic beads can be used to study interactions between proteins, such as antibodies and antigens, enzymes and substrates.
- Medical research : Protein precoupled magnetic beads can be used to study the mechanism of disease, such as cancer, autoimmune diseases, etc. For example, certain diseases can be diagnosed by isolating and analyzing specific antibodies in a patient's blood.
- Drug development : Protein precoupled magnetic beads can be used to screen drug targets and evaluate the biological activity of drugs.
- Chemical biology : Protein precoupled magnetic beads can be used to study protein structure and function, as well as protein interactions with small molecules.
Case Study
Case Study 1: Recombinant Mouse MMP9 Protein
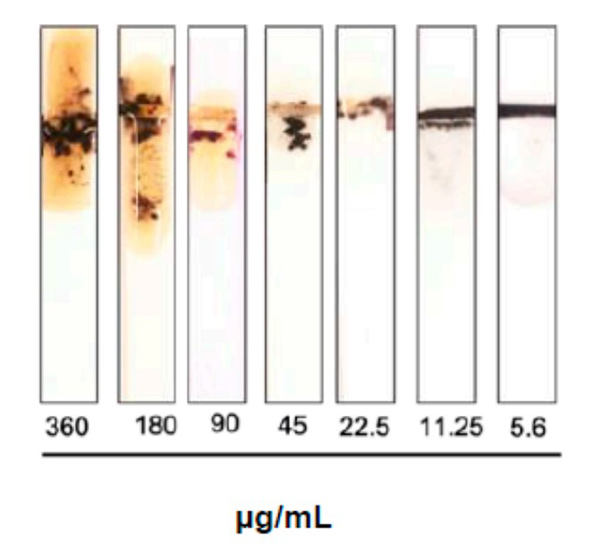
Sepsis is an immune response to a microbial invasion that causes organ injury and dysfunction due to a systemic inflammatory response. Sepsis is a serious, life-threatening condition and a widely recognized global health challenge. Given its high death rate, it is critical to diagnose sepsis and start treatment as early as possible. There is an urgent need for a sensitive and rapid screening method for detecting sepsis. In this study, the researchers investigated the use of MMP-9 as a biomarker for sepsis. A colorimetric paper-based biosensor was used for the detection of MMP-9 utilizing peptide-magnetic nanoparticle conjugates. The method is based on the cleavage of the MMP-9-specific peptide by the protease leading to the detaching of the magnetic beads from the sensor surface and changing of color. A fecal intraperitoneal (FIP) challenge was used to induce sepsis in mice, and an MMP-9 secretion was measured by taking blood and Bronchoalveolar Lavage (BAL) fluid samples at 1 h, 2 h, 4 h, and 20 h (early sepsis) post-challenge intervals. The results of the paper-based sensor for the detection of MMP-9 levels in blood samples and BAL samples were compared with ELISA and Western Blot.
(Nuha Khalid Alekhmimi, 2023)
Fig1. Image of the MMP-9 protease sensor responses for different blood samples taken from injected mice with different fecal concentrations.
Case Study 2: Recombinant Human PGP Protein
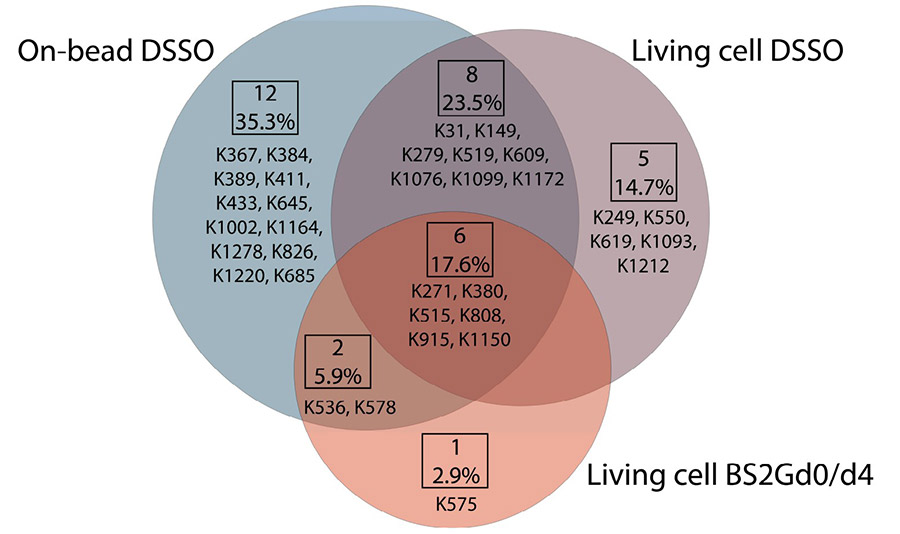
The ABC transporter P-glycoprotein (Pgp) has been found to be involved in multidrug resistance in tumor cells. Lipids and cholesterol have a pivotal role in Pgp's conformations; however, it is often difficult to investigate it with conventional structural biology techniques. On-bead, subsequent to membrane preparation and immunoprecipitation. Pgp-containing complexes were enriched employing extracellular monoclonal anti-Pgp antibodies on magnetic beads, followed by on-bead enzymatic digestion. The LC-MS/MS results revealed mono-links on Pgp's solvent-accessible residues, while intraprotein cross-links confirmed a complex interplay between extracellular, transmembrane, and intracellular segments of the protein, of which several have been reported to be connected to cholesterol. Harnessing the MS results and those of molecular docking, we suggest an epitope for the 15D3 cholesterol-dependent mouse monoclonal antibody.
(Gabriella Gellen, 2023)
Fig2. Overlap of unique mono-links on P-glycoprotein in different cross-linking setups.
Case Study 3: Recombinant SARS-CoV-1 SP Protein
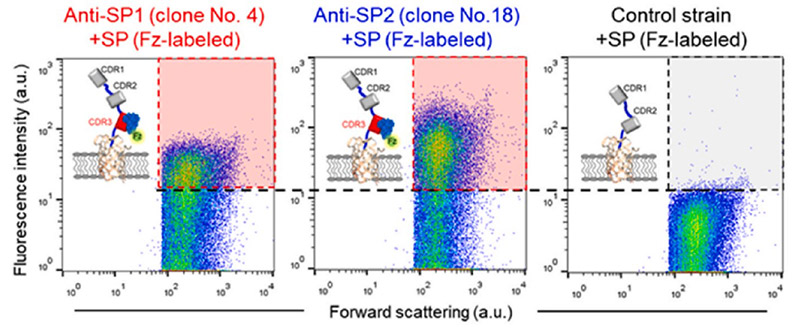
The detection of severe acute respiratory syndrome coronavirus (SARS-CoV-1) was demonstrated using screened Fv-antibodies for SPR biosensor and impedance spectrometry. The Fv-antibody library was first prepared on the outer membrane of E. coli using autodisplay technology and the Fv-variants (clones) with a specific affinity toward the SARS-CoV-1 spike protein (SP) were screened using magnetic beads immobilized with the SP. Upon screening the Fv-antibody library, two target Fv-variants (clones) with a specific binding affinity toward the SARS-CoV-1 SP were determined and the Fv-antibodies on two clones were named "Anti-SP1" (with CDR3 amino acid sequence: 1GRTTG5NDRPD11Y) and "Anti-SP2" (with CDR3 amino acid sequence: 1CLRQA5GTADD11V). The binding affinities of the two screened Fv-variants (clones) were analyzed using flow cytometry and the binding constants (KD) were estimated to be 80.5 ± 3.6 nM for Anti-SP1 and 45.6 ± 8.9 nM for Anti-SP2 (n = 3). In addition, the Fv-antibody including three CDR regions (CDR1, CDR2, and CDR3) and frame regions (FRs) between the CDR regions was expressed as a fusion protein (Mw. 40.6 kDa) with a green fluorescent protein (GFP) and the KD values of the expressed Fv-antibodies toward the SP estimated to be 15.3 ± 1.5 nM for Anti-SP1 (n = 3) and 16.3 ± 1.7 nM for Anti-SP2 (n = 3). Finally, the expressed Fv-antibodies screened against SARS-CoV-1 SP (Anti-SP1 and Anti-SP2) were applied for the detection of SARS-CoV-1.
(Jaeyong Jung, 2023)
Fig3. Flow cytometric analysis after treatment of the fluorescence labeled SP with the screened clones compared with the control strain with CDR1 and CDR2.
Case Study 4: Recombinant SARS-CoV-1 SP Protein
Protein modification plays an essential role in biological and pharmaceutical research. Due to the ordinary selectivity and inevitable damage to proteins of chemical synthetic methods, increased efforts were focused on biocatalysts which exhibited high regioselectivity and mild reaction conditions. However, separation of the biocatalysts and modified proteins remained a problem, especially when scaling up. Here, the researchers developed a simple method for site-specific protein modification with a recyclable biocatalyst. The immobilizing tyrosinase (BmTYR) on magnetic beads can oxidize C-terminal tyrosine residues of the target protein to o-quinone, followed by the spontaneous addition of different nucleophiles (e.g., aniline derivatives), resulting in a C-terminal modified protein. Compared to the homogeneous biocatalytic system reported before, this heterogeneous system leads to an easier separation. Furthermore, the solid-phase biocatalyst can be regenerated during separation, providing reusability and lower costs.
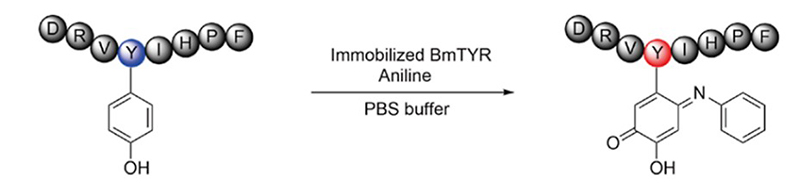
(Xingyu Ji, 2022)
Fig4. Peptides Modified with Aniline Using Immobilized BmTYR. Angiotensin II modification at 1.5 equiv aniline.
Advantages
- Professional technical ability : Our research team has strong professional knowledge and skills in biology, chemistry and other disciplines, and can master the preparation, application and analysis of protein precoupled magnetic beads.
- Perfect service system : We can provide a full range of services, including preliminary consultation, experimental design, data analysis and result reporting, to ensure that customers get high-quality service experience.
- Rich experience : We have sufficient practical experience, according to the needs of customers and sample characteristics, to develop appropriate experimental programs and operating processes.
- Wide variety : Creative BioMart especially provides over 26,000 protein pre-coupled magnetic beads, which coupled with more than 10,000 different proteins types.
FAQ
-
Q: How to guarantee products quality?
A: We evaluate the purity, specificity and stability of the provided magnetic beads from multiple angles, and issue a complete data analysis report.
-
Q: In what areas can the product be used for research?
A: We offer products that can be used for, but are not limited to, immune capture, biopanning, sample/cell enrichment, analytical laboratory sample pretreatment, flow cytometry.
Resources
-

Introducing Our Perfect Protein Pre Coupled Magnetic Beads Products
-
JAK-STAT Signaling Pathway Related Proteins-Whole Set Tools for Pathway Research
-

Creative BioMart IP/Co-IP Magnetic Beads — A New Gold Standard for Protein Interaction Research
References
- Alekhmimi NK.; et al . Paper-Based Biosensor for the Detection of Sepsis Using MMP-9 Biomarker in FIP Mice Model. Biosensors (Basel) . 2023;13(8):804.