Lipid/Cholesterol Metabolism
Creative BioMart Lipid/Cholesterol Metabolism Product List
Immunology Background
Background
Lipid metabolism encompasses the processes by which lipids are synthesized, modified, degraded, and utilized in living organisms. Lipids, which include fats, oils, waxes, steroids, and other related compounds, are hydrophobic or amphipathic molecules that are critical for energy storage, cellular structure, and signaling. Cholesterol, a sterol and lipid, plays a vital role in the integrity of cell membranes and serves as a precursor for steroid hormones, bile acids, and vitamin D.
Metabolism Process
Lipid Digestion and Absorption
Dietary lipids, primarily triglycerides, are digested in the small intestine by bile salts and pancreatic lipase. Bile salts emulsify fat droplets, increasing their surface area for enzyme action. Pancreatic lipase hydrolyzes triglycerides into monoglycerides and free fatty acids, which are absorbed by enterocytes. Within the enterocytes, these lipids are re-esterified into triglycerides and packaged into chylomicrons for transport through the lymphatic system and into the bloodstream.
Lipoprotein Transport
Lipoproteins are complexes of lipids and proteins that transport lipids through the aqueous environment of the bloodstream. Chylomicrons transport dietary triglycerides and cholesterol from the intestines to peripheral tissues. Very-low-density lipoproteins (VLDL), produced by the liver, transport endogenous triglycerides. As VLDL triglycerides are hydrolyzed by lipoprotein lipase (LPL) in peripheral tissues, VLDL is converted to intermediate-density lipoproteins (IDL) and then to low-density lipoproteins (LDL), which are rich in cholesterol. High-density lipoproteins (HDL) are involved in reverse cholesterol transport, carrying excess cholesterol from tissues back to the liver for excretion or recycling.
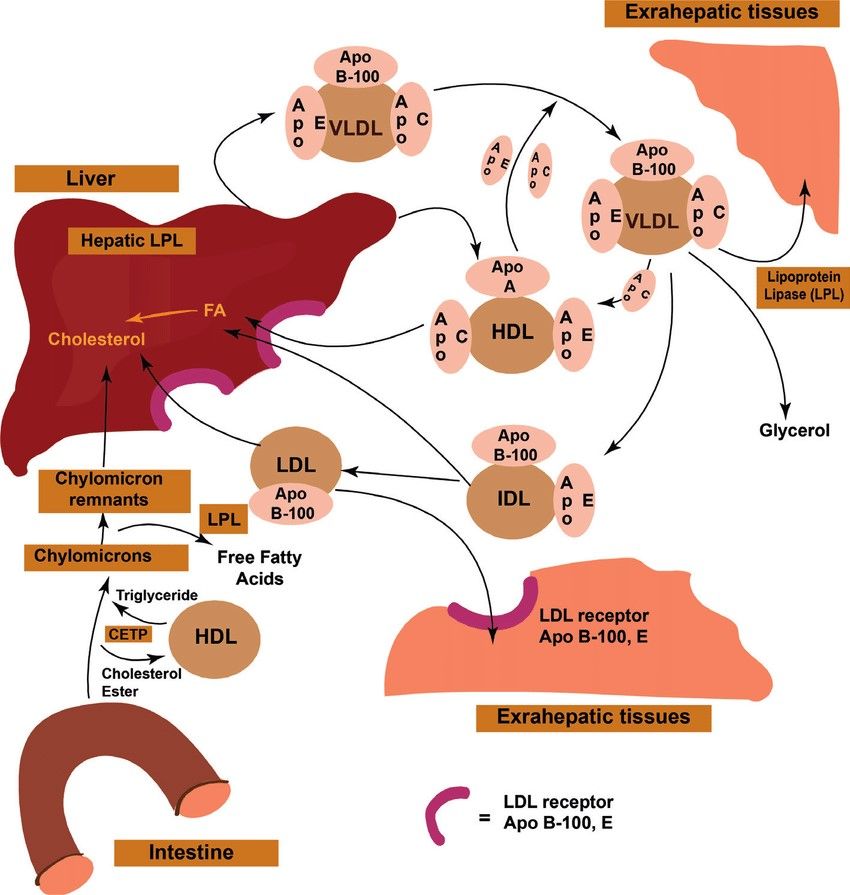 Fig. 1: Lipid transport and storage (Zhyvotovska et al., 2019).
Fig. 1: Lipid transport and storage (Zhyvotovska et al., 2019).β-Oxidation and Ketogenesis
Fatty acid catabolism occurs primarily in the mitochondria through β-oxidation. Fatty acids are activated to fatty acyl-CoA and transported into the mitochondrial matrix via the carnitine shuttle. β-oxidation involves the sequential removal of two-carbon units as acetyl-CoA, generating NADH and FADH2, which enter the electron transport chain to produce ATP. Acetyl-CoA produced by β-oxidation can also enter the citric acid cycle for further oxidation.
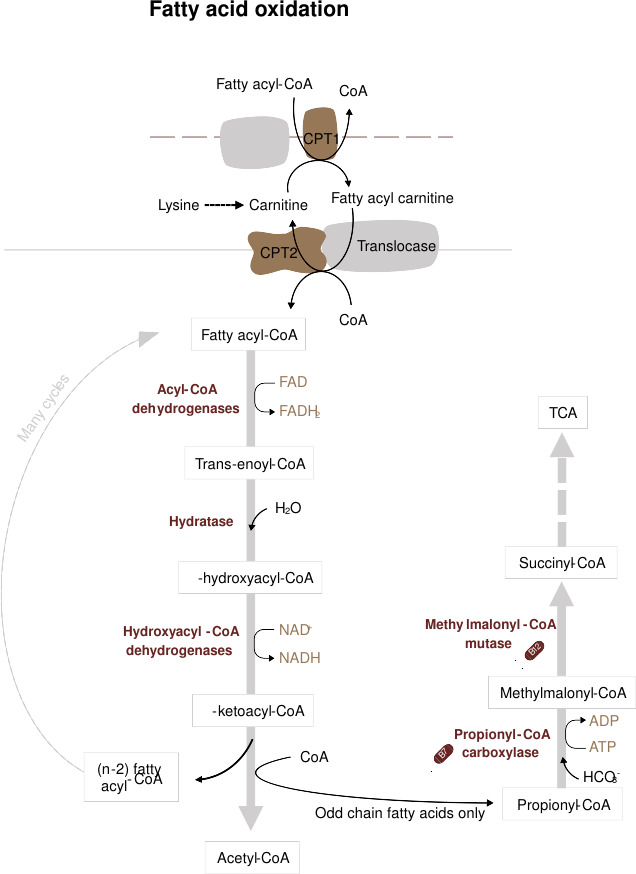 Fig. 2: Overview of long-chain fatty acids (LCFA) transport into the mitochondria and β-oxidation (Grey, 2021).
Fig. 2: Overview of long-chain fatty acids (LCFA) transport into the mitochondria and β-oxidation (Grey, 2021).During periods of fasting or low carbohydrate intake, acetyl-CoA is diverted to ketogenesis in the liver, producing ketone bodies (acetoacetate, β-hydroxybutyrate, and acetone) that serve as an alternative energy source for peripheral tissues, including the brain.
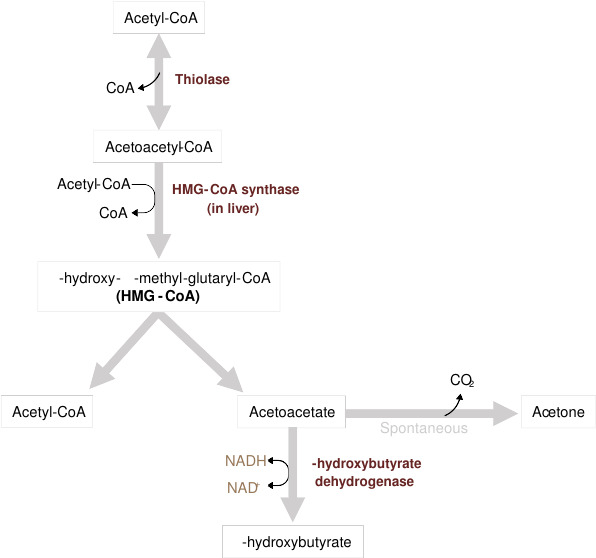 Fig. 3: Overview of ketone body formation (Grey, 2021).
Fig. 3: Overview of ketone body formation (Grey, 2021).Physiological Roles of Lipid Metabolism
Energy Storage and Utilization
Triglycerides stored in adipose tissue serve as a major energy reservoir. During periods of energy demand, lipolysis breaks down triglycerides into free fatty acids and glycerol, which are released into the bloodstream. Free fatty acids are taken up by peripheral tissues and oxidized for ATP production, while glycerol is converted to glucose in the liver via gluconeogenesis.
Cellular Structure
Phospholipids and cholesterol are essential components of cellular membranes, contributing to membrane fluidity, integrity, and function. Phospholipids form the bilayer structure, while cholesterol modulates membrane fluidity and serves as a precursor for biologically active molecules.
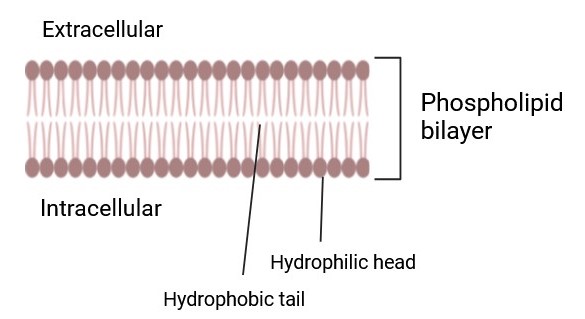 Fig. 4: Phospholipid Bilayer (Created with BioRender.com).
Fig. 4: Phospholipid Bilayer (Created with BioRender.com).Signal Transduction
Lipid-derived molecules play a critical role in signal transduction. Phosphoinositides are involved in intracellular signaling pathways, while arachidonic acid-derived molecules (prostaglandins, thromboxanes, leukotrienes) regulate inflammation, immunity, and other physiological processes.
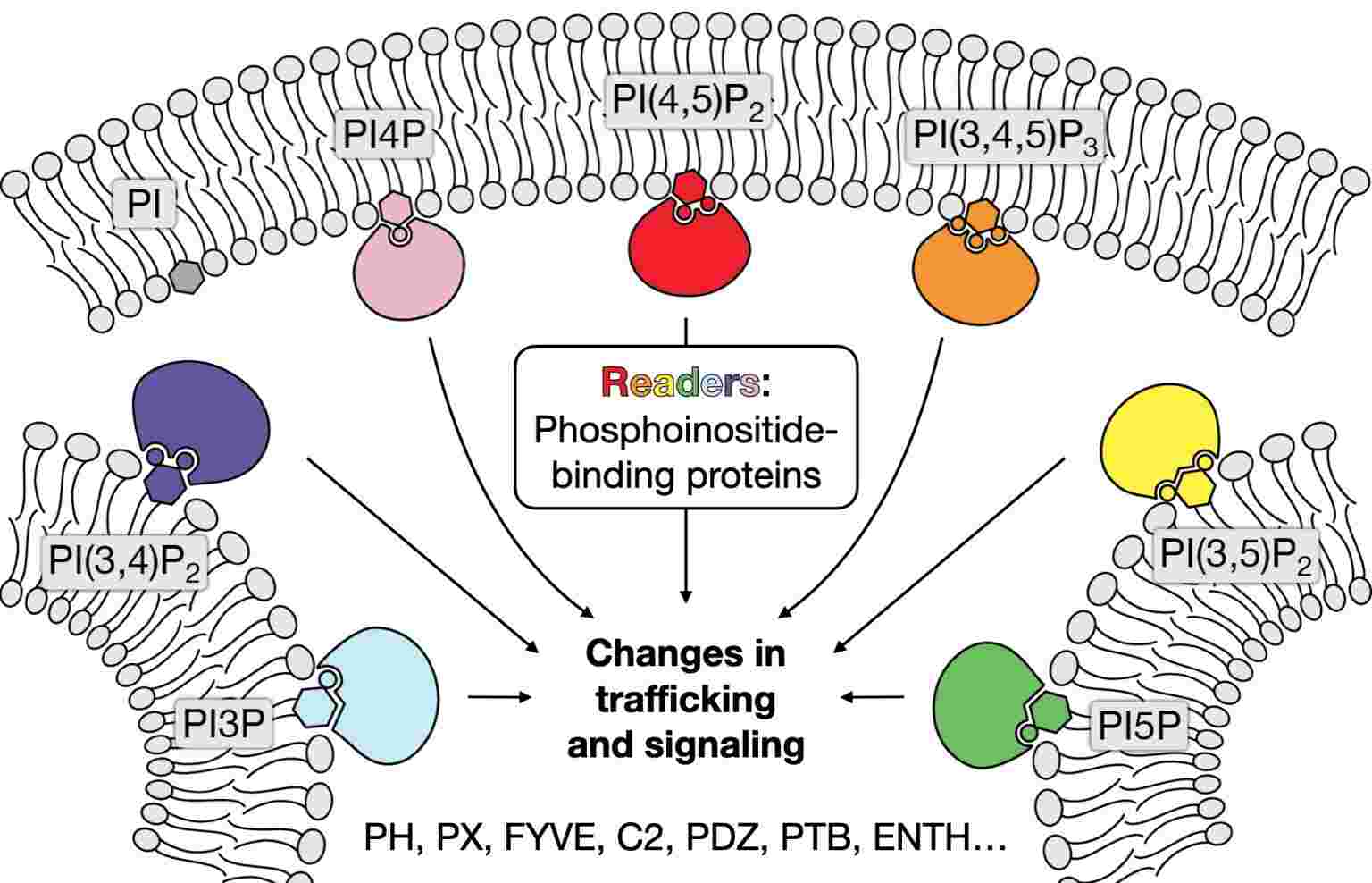 Fig. 5: Simplified schematic of phosphoinositides involved in signaling pathway (The Baskin Lab, Cornell University).
Fig. 5: Simplified schematic of phosphoinositides involved in signaling pathway (The Baskin Lab, Cornell University).Hormone Production
Cholesterol is the precursor of steroid hormones, including glucocorticoids, mineralocorticoids, and sex hormones. These hormones regulate a wide range of physiological functions, including metabolism, stress response, electrolyte balance, and reproduction.
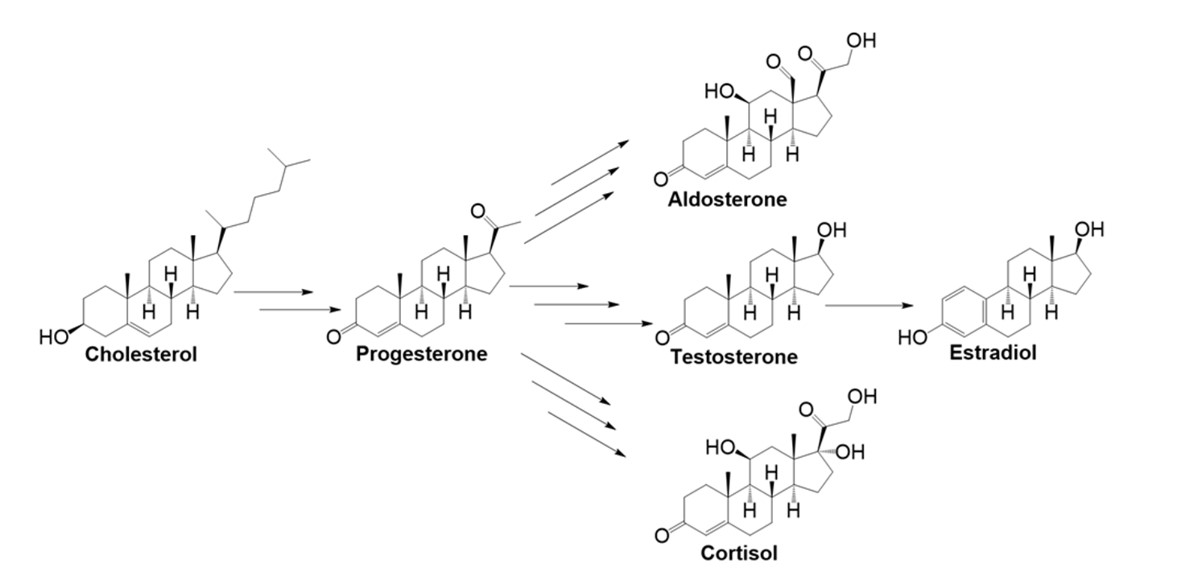 Fig. 6: The five major endogenous steroid hormones derived from cholesterol: progesterone, aldosterone, testosterone, estradiol, and cortisol (Pflug, 2017).
Fig. 6: The five major endogenous steroid hormones derived from cholesterol: progesterone, aldosterone, testosterone, estradiol, and cortisol (Pflug, 2017).Bile Acid Synthesis
Bile acids, synthesized from cholesterol in the liver, are essential for the digestion and absorption of dietary fats and fat-soluble vitamins. They act as detergents, emulsifying dietary lipids to facilitate their enzymatic breakdown and absorption in the intestine.
Detoxification
Lipid metabolism in the liver includes detoxification of xenobiotics and endogenous waste products. Lipid-soluble toxins are often metabolized to more water-soluble forms for excretion in the bile or urine.
Regulation of Lipid Metabolism
Lipid metabolism is tightly regulated to maintain energy homeostasis and ensure proper cellular function. This regulation occurs at multiple levels, including transcriptional, post-transcriptional, and post-translational modifications, as well as through hormonal and nutritional signals. Here are the key mechanisms involved in the regulation of lipid metabolism:
1. Hormonal Regulation
Insulin promotes lipid storage and synthesis. Specifically, insulin activates lipoprotein lipase (LPL) in adipose tissue, increasing the uptake of fatty acids from chylomicrons and VLDL. It also stimulates acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) in the liver and adipose tissue, promoting fatty acid synthesis. Insulin signaling through the PI3K-Akt pathway leads to the activation of SREBP-1c (Sterol Regulatory Element-Binding Protein-1c), a key transcription factor that upregulates genes involved in lipid synthesis. SREBP1c and ChREBP activate lipogenic genes such as FASN, ACC, Scd1 and Elovl6. At low cellular ATP levels, activation of AMPK interacts with and phosphorylates SREBP1c, thus inhibiting proteolytic cleavage and nuclear translocation, and repressing DNL.
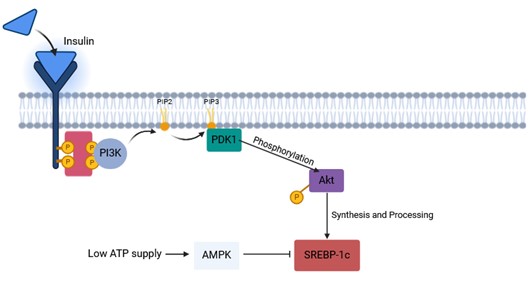 Fig. 7: Mechanism of insulin signaling pathway. Upon insulin receptors binding to ligands, leading to self-phosphorylation, insulin receptor substrates (IRS) are recruited and phosphorylated. IRS proteins are then recruited and activate PI3K-Akt pathway through their activation are enhanced both the synthesis and processing of SREBP (SREBP1c mainly) in hepatocytes (Created with BioRender.com).
Fig. 7: Mechanism of insulin signaling pathway. Upon insulin receptors binding to ligands, leading to self-phosphorylation, insulin receptor substrates (IRS) are recruited and phosphorylated. IRS proteins are then recruited and activate PI3K-Akt pathway through their activation are enhanced both the synthesis and processing of SREBP (SREBP1c mainly) in hepatocytes (Created with BioRender.com).Glucagon and epinephrine promote lipid breakdown and mobilization. These hormones activate hormone-sensitive lipase (HSL) in adipose tissue through the cAMP-PKA pathway, resulting in the hydrolysis of triglycerides to free fatty acids and glycerol. Glucagon and epinephrine reduce the activity of ACC, thereby decreasing fatty acid synthesis.
2. Transcriptional Regulation
SREBPs (Sterol Regulatory Element-Binding Proteins) are key regulators of lipid biosynthesis. SREBPs are synthesized as inactive precursors bound to the endoplasmic reticulum. When cholesterol levels are low, SREBPs are transported to the Golgi apparatus, where they are cleaved to release the active form. The active SREBPs enter the nucleus and upregulate genes involved in cholesterol and fatty acid synthesis.
PPARs (Peroxisome Proliferator-Activated Receptors) regulate lipid catabolism and storage. PPARs are nuclear receptors that, upon binding to fatty acids or their derivatives, heterodimerize with RXR (retinoid X receptor) and bind to PPAR response elements in the promoter regions of target genes. There are three types of PPARs: PPARα (fatty acid oxidation in liver and muscle), PPARγ (adipogenesis and lipid storage in adipose tissue), PPARδ/β (fatty acid oxidation and energy expenditure).
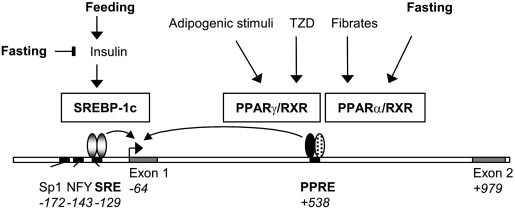 Fig. 8: Model for the dual regulation of ACBP expression by PPARs and SREBPs. In rodent hepatocytes the ACBP gene is regulated by insulin via the induction of SREBP-1c activity. SREBP-1c activates expression by binding to an SRE in the proximal promoter, and the transcription factors NF-Y and Sp1 function as auxiliary factors. PPAR/RXR activates ACBP expression in response to fibrates and other PPAR activators by binding to the PPRE in intron 1. Fasting leads to inhibition of SREBP-1c activity but induces PPAR activity. In adipocytes and other cells that express PPAR, ACBP expression is activated by thiazolidinediones (TZDs) and other PPAR activators through PPAR/RXR binding to the intronic PPRE. Position of regulatory elements and 5 end of exons relative to the translation start site are indicated in italics. See text for further details (Sandberg et al., 2005).
Fig. 8: Model for the dual regulation of ACBP expression by PPARs and SREBPs. In rodent hepatocytes the ACBP gene is regulated by insulin via the induction of SREBP-1c activity. SREBP-1c activates expression by binding to an SRE in the proximal promoter, and the transcription factors NF-Y and Sp1 function as auxiliary factors. PPAR/RXR activates ACBP expression in response to fibrates and other PPAR activators by binding to the PPRE in intron 1. Fasting leads to inhibition of SREBP-1c activity but induces PPAR activity. In adipocytes and other cells that express PPAR, ACBP expression is activated by thiazolidinediones (TZDs) and other PPAR activators through PPAR/RXR binding to the intronic PPRE. Position of regulatory elements and 5 end of exons relative to the translation start site are indicated in italics. See text for further details (Sandberg et al., 2005).3. Nutritional Regulation
Dietary fat intake affects lipid metabolism based on the quantity and quality of fat. High dietary fat intake increases chylomicron production and VLDL secretion. Saturated and trans fats can increase LDL cholesterol levels, while unsaturated fats, especially omega-3 fatty acids, can increase HDL cholesterol levels and decrease triglycerides. Dietary fats influence the expression of genes involved in lipid metabolism through PPARs and SREBPs.
Carbohydrate intake affects de novo lipogenesis (DNL). High carbohydrate intake increases insulin secretion, which stimulates DNL in the liver. Excess carbohydrates are converted to fatty acids and stored as triglycerides.
4. Post-Translational Modifications
Phosphorylation modulates enzyme activity. Enzymes involved in lipid metabolism, such as HSL and ACC, are regulated by phosphorylation. For example, PKA phosphorylates HSL and activates it to hydrolyze triglycerides. Conversely, phosphorylation of ACC by AMP-activated protein kinase (AMPK) inhibits its activity, thereby reducing fatty acid synthesis.
Acetylation and ubiquitination affect the stability and activity of proteins. Acetylation can modulate the activity of transcription factors such as SREBPs. Ubiquitination targets proteins for degradation by the proteasome, thereby regulating the turnover of key enzymes involved in lipid metabolism.
5. Feedback Mechanisms
Cholesterol feedback maintains cholesterol homeostasis. High cellular cholesterol levels inhibit the activation of SREBPs, reducing cholesterol synthesis and uptake. This involves the binding of cholesterol to SCAP (SREBP cleavage-activating protein), preventing its transport to the Golgi. Cholesterol activates liver X receptors (LXRs), which upregulate genes involved in cholesterol efflux and catabolism.
6. Circadian Regulation
Circadian rhythms synchronize lipid metabolism with the body's daily cycle. The expression of enzymes and hormones involved in lipid metabolism follows a circadian pattern. Clock genes, such as CLOCK and BMAL1, regulate the rhythmic expression of genes involved in lipid metabolism. Disruption of circadian rhythms, such as shift work or irregular eating patterns, can lead to metabolic disorders, including dyslipidemia and obesity.
Related Disease
Dysregulation of lipid metabolism can lead to a variety of diseases, many of which are major contributors to morbidity and mortality worldwide. Here are some of the key diseases associated with lipid metabolism disorders:
1. Obesity
Obesity is characterized by excessive fat accumulation due to an imbalance between caloric intake and energy expenditure. Dysregulation of lipid storage and catabolism leads to increased adipose tissue mass. In addition, insulin resistance often leads to obesity. Obesity increases the risk of type 2 diabetes, cardiovascular disease, certain cancers, and metabolic syndrome.
2. Type 2 Diabetes Mellitus
Type 2 diabetes is a metabolic disorder characterized by hyperglycemia due to insulin resistance and relative insulin deficiency. Insulin resistance affects lipid metabolism, leading to elevated free fatty acids, increased triglyceride synthesis, and altered lipoprotein profiles. Complications of type 2 diabetes include macrovascular complications (e.g., coronary artery disease) and microvascular complications (e.g., neuropathy, nephropathy).
3. Atherosclerosis and Cardiovascular Disease
Atherosclerosis is the buildup of plaque in the artery walls that leads to cardiovascular diseases such as coronary heart disease, stroke, and peripheral artery disease. Elevated levels of LDL cholesterol and low levels of HDL cholesterol contribute to plaque formation. And oxidized LDL cholesterol causes inflammation and endothelial dysfunction. Severe cases can lead to heart attack, stroke and other cardiovascular events.
4. Non-Alcoholic Fatty Liver Disease (NAFLD)
NAFLD is characterized by excessive accumulation of fat in the liver in the absence of significant alcohol consumption. Dysregulation of lipid metabolism leads to increased hepatic lipid synthesis and decreased lipid export. NAFLD can progress to non-alcoholic steatohepatitis (NASH), fibrosis and cirrhosis.
5. Metabolic Syndrome
Metabolic syndrome is a cluster of conditions including central obesity, insulin resistance, dyslipidemia (high triglycerides and low HDL cholesterol), and hypertension. Central obesity and insulin resistance lead to dysregulated lipid metabolism, contributing to the other components of the syndrome.
6. Hyperlipidemia and Dyslipidemia
Hyperlipidemia is an abnormally high concentration of lipids in the blood, while dyslipidemia refers to abnormal lipid profiles, including high LDL cholesterol, low HDL cholesterol, and high triglycerides. Genetic and lifestyle factors contribute to abnormal lipid metabolism. For example, familial hypercholesterolemia is a genetic disorder characterized by very high levels of LDL cholesterol due to defective LDL receptors.
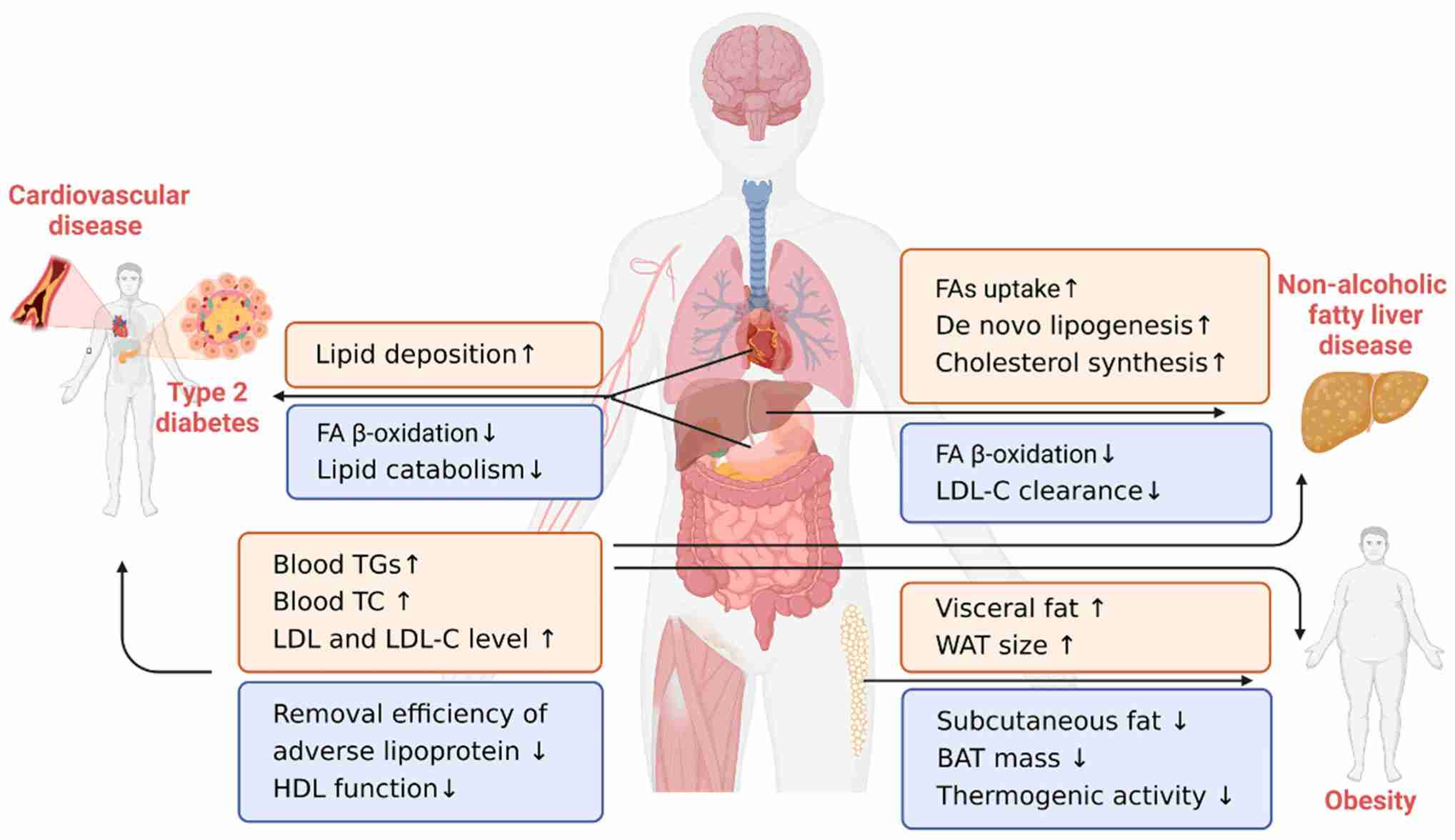 Fig. 9: Lipid-related chronic diseases (Song et al., 2023).
Fig. 9: Lipid-related chronic diseases (Song et al., 2023).Case Study
Case 1: Russell, David W. The enzymes, regulation, and genetics of bile acid synthesis. Annual Review of Biochemistry, vol. 72, no. 1, June 2003, pp. 137–74.
Increasing evidence suggests that cancer cells exhibit distinct changes in various facets of lipid metabolism. Alterations in lipid metabolism can impact several cellular processes, such as cell growth, proliferation, differentiation, and motility.
Certain alterations in lipid metabolism may contribute to the predisposition of obese patients to develop cancer. Obesity and insulin resistance may contribute to cancer development by increasing the secretion of insulin by pancreatic beta cells and by increasing the availability of IGF1 as a result of increased production of IGF-binding proteins. Adipose tissue secretion of inflammatory cytokines may also promote tumor cell transformation and proliferation. On the other hand, tumor burden promotes the degradation of lipids in the adipose tissue of cachexic patients, and tumor cells may use circulating free fatty acids for energy, membrane biosynthesis, or signaling. Glycerol produced by the degradation of triacylglycerides can be used for gluconeogenesis in the liver.
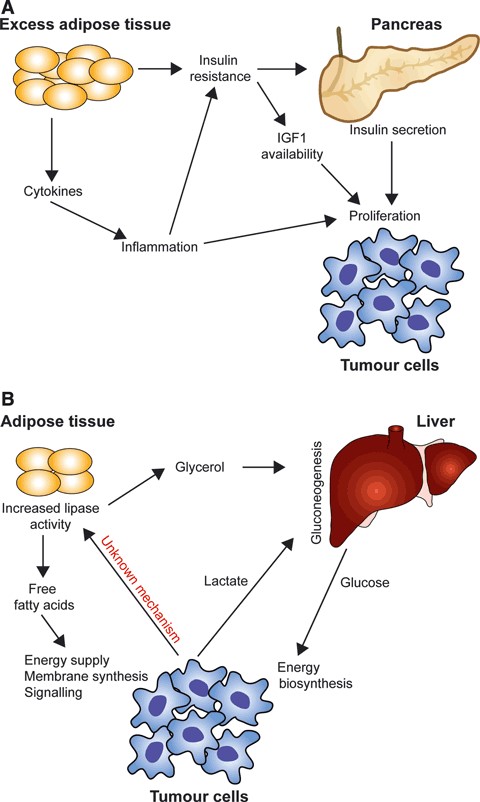 Fig. 10: Whole-body lipid metabolism and cancer. (A) Obesity and insulin resistance affect cancer development. (B) tumor cells promote lipid breakdown in adipose tissue of patients with cachexia (Santos and Schulze, 2012).
Fig. 10: Whole-body lipid metabolism and cancer. (A) Obesity and insulin resistance affect cancer development. (B) tumor cells promote lipid breakdown in adipose tissue of patients with cachexia (Santos and Schulze, 2012).Case 2: Wu, X.; et al. Lipid metabolism in prostate cancer. Am J Clin Exp Urol. 2014 Jul 12;2(2):111-20.
Dysregulation of lipid metabolism is a hallmark of the malignant phenotype; increased lipid accumulation secondary to alterations in the levels of a variety of lipid metabolic enzymes has been documented in a variety of tumors, including prostate. Alterations in prostate lipid metabolism include upregulation of several lipogenic enzymes as well as enzymes that function to oxidize fatty acids as an energy source. Cholesterol and phospholipid metabolism are also affected. There is evidence that lipid synthesis is increased in prostate cancer.
In this study, the researchers summarize data from several representative studies showing the fold change and statistical significance of overexpression of several lipogenic enzyme mRNAs (Table 1) in human tumor samples.
Tab. 1: Lipogenic enzyme mRNA expression in normal and cancerous human prostate.
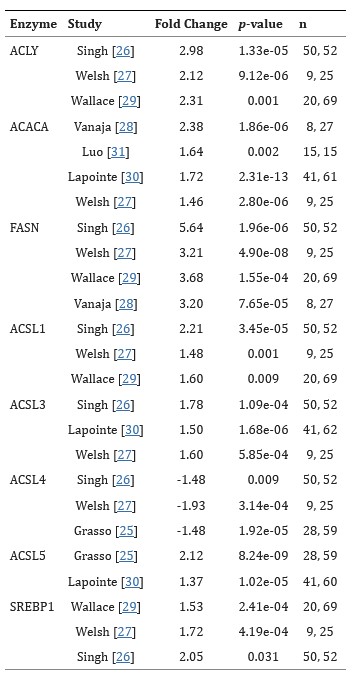
Fold change = cancer/normal. n = number of samples (normal, cancer). Data taken from Oncomine. Numbered studies refer to the original references in this study.
References
- Kindred Grey (2021). "Overview of LCFA transport into the mitochondria and β-oxidation."
- Kindred Grey (2021). "Overview of ketone body formation."
- Nicholas C. Pflug. Identification of Bioactive Products from Environmental Transformation of Steroids. 2017. ETH Zurich.
- "Phosphoinositide Signaling in Cell Biology and Cancer." The Baskin Lab Weill Institute for Cell & Molecular Biology, https://pubs.acs.org/doi/10.1021/acschembio.2c00527.
- Russell, David W. "The Enzymes, Regulation, and Genetics of Bile Acid Synthesis." Annual Review of Biochemistry, vol. 72, no. 1, June 2003, pp. 137–74. DOI.org (Crossref), https://doi.org/10.1146/annurev.biochem.72.121801.161712.
- Sandberg, Maria B., et al. "The Gene Encoding Acyl-CoA-Binding Protein Is Subject to Metabolic Regulation by Both Sterol Regulatory Element-Binding Protein and Peroxisome Proliferator-Activated Receptor α in Hepatocytes." Journal of Biological Chemistry, vol. 280, no. 7, Feb. 2005, pp. 5258–66. DOI.org (Crossref), https://doi.org/10.1074/jbc.M407515200.
- Santos, Claudio R., and Almut Schulze. "Lipid Metabolism in Cancer." The FEBS Journal, vol. 279, no. 15, Aug. 2012, pp. 2610–23. DOI.org (Crossref), https://doi.org/10.1111/j.1742-4658.2012.08644.x.
- Song, Rui, et al. "The Roles of Lipid Metabolism in the Pathogenesis of Chronic Diseases in the Elderly." Nutrients, vol. 15, no. 15, Jan. 2023, p. 3433. www.mdpi.com, https://doi.org/10.3390/nu15153433.
- Wu, Xinyu, et al. "Lipid Metabolism in Prostate Cancer." American Journal of Clinical and Experimental Urology, vol. 2, no. 2, July 2014, pp. 111–20. PubMed Central, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4219300/.
- Zhang, Dengke, et al. "Important Hormones Regulating Lipid Metabolism." Molecules, vol. 27, no. 20, Oct. 2022, p. 7052. PubMed Central, https://doi.org/10.3390/molecules27207052.
- Zhyvotovska, Angelina, et al. "Introductory Chapter: Overview of Lipoprotein Metabolism." Dyslipidemia, edited by Samy I. McFarlane, IntechOpen, 2019. DOI.org (Crossref), https://doi.org/10.5772/intechopen.85094.

