Cancer Biomarkers
Creative BioMart Cancer Biomarkers Product List
- Bladder Cancer Biomarkers
- Breast Cancer Biomarkers
- Cancer Biomarkers in Clinical Practice
- Colon Cancer Biomarkers
- Gastric Cancer Biomarkers
- Glioma Biomarkers
- Head and Neck Cancer Biomarkers
- Liver Cancer Biomarkers
- Lung Cancer Biomarkers
- Medulloblastoma Biomarkers
- Melanoma Biomarkers
- Osteosarcoma Biomarkers
- Ovarian Cancer Biomarkers
- Pancreatic Cancer Biomarkers
- Prostate Cancer Biomarkers
Immunology Background
Background
Cancer remains one of the greatest challenges in modern medicine, causing millions of deaths worldwide each year. One of the most promising approaches to improve cancer diagnosis, treatment and prognosis is the study and application of cancer biomarkers (Biomarker Service). These biological molecules that indicate the presence or progression of cancer have revolutionized our understanding and management of this complex disease.
Definition of Cancer Biomarkers
A biomarker, or biological marker, is a measurable indicator of a biological state or condition. In the context of cancer, biomarkers are molecules produced either by the tumor itself or by other tissues in response to the presence of cancer. They can include a wide range of substances such as proteins, nucleic acids, antibodies and metabolites. Biomarkers can be found in blood, urine, tissue, or other body fluids and can provide information about the presence, type, progression, and response to treatment of cancer.
Cancer biomarkers are divided into several categories based on their application: diagnostic, prognostic, and predictive. Diagnostic biomarkers help detect the presence of cancer. Prognostic biomarkers provide information about the likely course of the disease, regardless of treatment. Predictive biomarkers predict how well a cancer patient will respond to a particular treatment, helping to personalize treatment plans.
Classification of Cancer Markers
There are several ways to classify cancer markers.
1. A traditionally accepted way of classification is into:
- Oncofetal antigens (Carcinoembryonic antigen CEA, Alpha-fetoprotein AFP)
- Glycoprotein antigens or carbohydrate antigens (Cancer antigen CA-125, CA 19.9, CA 15-3)
- Enzymes (Alkaline phosphatase ALP, Neuron-specific enolase NSE)
- Hormone receptors (Estrogen receptor/progesterone receptor ER/PR)
- Hormones (β-human chorionic gonadotropin β-HCG, calcitonin)
- Other biomolecules (Serum vanillylmandelic acid VMA, 5-Hydroxyindoleacetic acid 5HIAA).
2. Tumor markers can also be classified based on:
- Biochemical structure
- Function
- Combination of biochemical structure and function
- Discovery of oncofetal antigens.
Important Cancer Biomarkers
Common Cancer Markers
- Carcinoembryonic Antigen (CEA)
CEA is a glycoprotein with a molecular weight of 200 kDa and is normally derived from embryonic endodermal epithelium in the fetus, controlled by fetal oncogenes. It usually disappears from serum after birth; however, small quantities of CEA may remain in colon tissue. CEA is a non-specific serum biomarker that is elevated in various malignancies such as colorectal cancer, medullary thyroid cancer, breast cancer, mucinous ovarian cancer, etc.
- Cancer Antigen 125 (CA-125)
The tumor marker CA-125 represents the protein mucin-16, also known as MUC-16. It is a long-chain transmembrane glycoprotein found in coelomic mesothelial cells such as those in the peritoneum, pericardium, and pleura. It is also expressed by epithelial cells of the Müllerian duct system, which includes the epithelium of the endometrium, inner cervix, and fallopian tubes. CA-125 is primarily used as a biomarker for ovarian cancer. While not specific for ovarian cancer alone, elevated CA-125 levels may indicate the presence of this disease and are useful in monitoring response to treatment and recurrence of diseases such as endometrial, breast and lung cancer.
- Cancer Antigen 15-3 (CA 15-3)
The CA 15-3 marker is most useful in evaluating the response to treatment in women with advanced breast cancer. Elevated levels of CA 15-3 are also associated with ovarian, lung, and prostate cancer, as well as non-cancerous conditions such as benign breast or ovarian disease, endometriosis, pelvic inflammatory disease, and hepatitis. Pregnancy and lactation can also increase CA 15-3 levels.
- Cancer Antigen 19-9 (CA 19-9)
The marker CA 19-9 is associated with cancers of the colon, stomach, and bile duct. Elevated levels of CA 19-9 may indicate advanced pancreatic cancer, but it is also associated with non-cancerous conditions, including gallstones, pancreatitis, cirrhosis of the liver, and cholecystitis.
- Alpha-fetoprotein (AFP)
AFP, also known as alpha foetoprotein or α1-foetoprotein, is a mammalian glycoprotein that is produced during human embryonic development during pregnancy by endodermal tissue and the fetal liver or, in adults, specifically by tumor cells of the liver. High levels of AFP can be a sign of liver, ovarian or testicular cancer.
- Lactate Dehydrogenase (LDH)
LDH is also a non-specific marker of tissue turnover, which is a normal metabolic process. Many cancers cause a general increase in LDH or an increase in one of its isoenzymes. Therefore, it may be a non-specific tumor marker that is not useful in identifying the type of cancer.
- KRAS
This gene, a Kirsten RAS oncogene homolog from the mammalian RAS gene family, encodes a protein that is a member of the small GTPase superfamily. Mutations in the KRAS gene are common in several cancers, including colorectal and lung cancer. KRAS mutations are often predictive of resistance to certain targeted therapies, making them important for treatment planning.
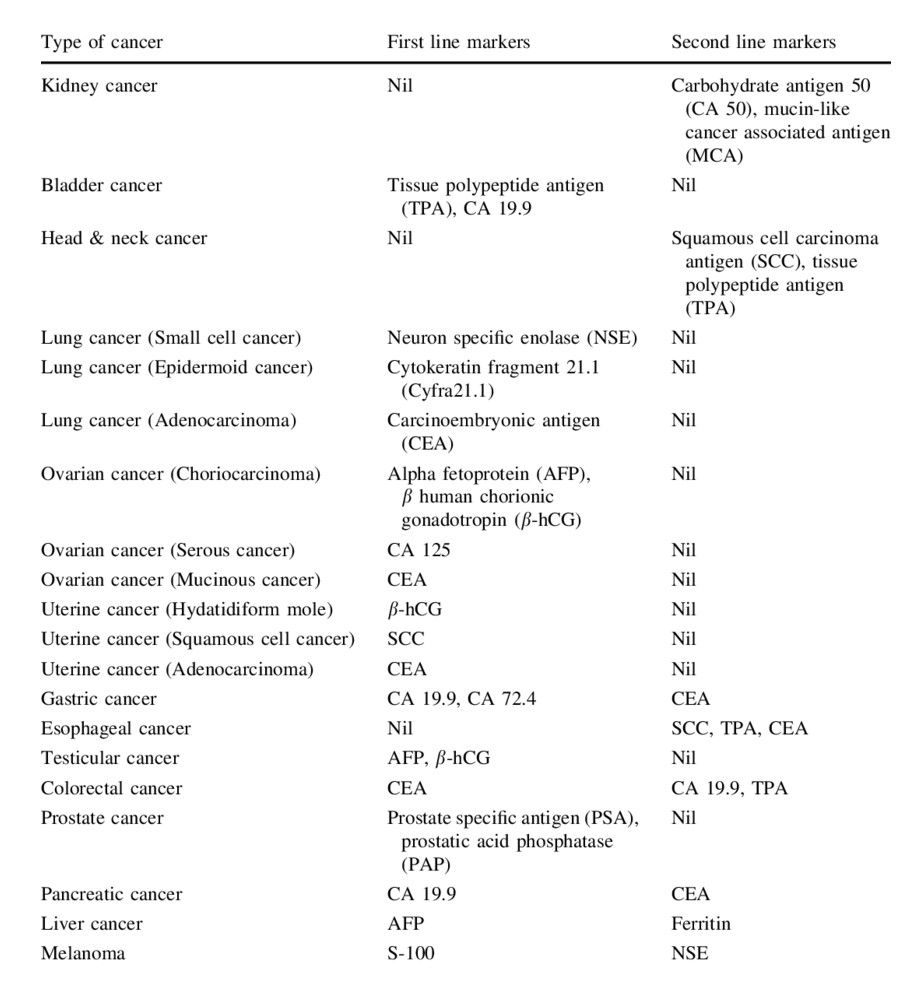
Traditionally used tumor markers in different types of cancers
In addition to common cancer biomarkers that are elevated in more than one type of cancer, there are a large number of tumor markers that are used for different types of cancer. A summary of traditional tumor markers is provided in Table 1.
Tab. 1: Traditionally used tumor markers in different types of cancers (Vaidyanathan and Vasudevan, 2012).
Detection of Cancer Biomarkers
Several sophisticated techniques are used to detect and measure cancer biomarkers, each with its advantages and limitations:
Enzyme-Linked Immunosorbent Assay (ELISA): This is a common method for detecting and quantifying proteins, including cancer biomarkers, in blood or other body fluids. ELISA is highly sensitive and specific, making it suitable for routine clinical use.
Polymerase Chain Reaction (PCR): PCR is a powerful technique for amplifying and detecting specific DNA or RNA sequences. It is widely used to identify genetic mutations associated with cancer, such as BRCA mutations or KRAS mutations.
Next-Generation Sequencing (NGS): NGS provides a comprehensive analysis of a patient's genome or specific genes. It can identify a wide range of genetic alterations in a single test, providing valuable information for personalized cancer treatment.
Immunohistochemistry (IHC): IHC uses antibodies to detect specific proteins in tissue samples. It is commonly used to assess the expression of biomarkers such as HER2 in breast cancer or PD-L1 in lung cancer.
Mass Spectrometry: This technique can identify and quantify proteins, peptides, and metabolites in complex biological samples. It is useful for the discovery of new biomarkers and the validation of known biomarkers.
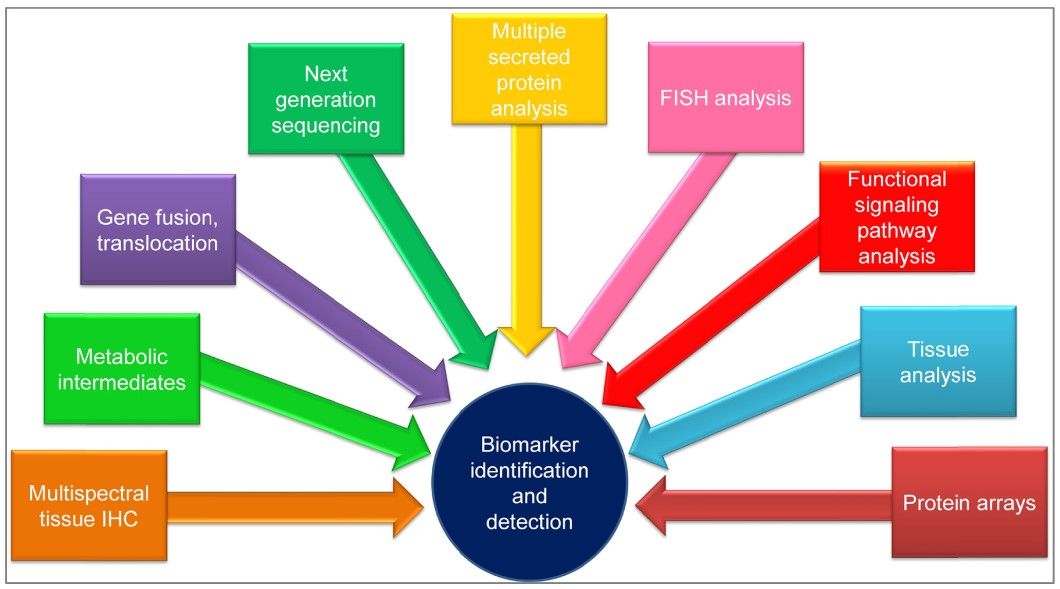 Fig. 1: Techniques used for biomarker identification and detection. Abbreviations: FISH, fluorescence in situ hybridization; IHC, immunohistochemistry (Tothill and Altintas, 2015).
Fig. 1: Techniques used for biomarker identification and detection. Abbreviations: FISH, fluorescence in situ hybridization; IHC, immunohistochemistry (Tothill and Altintas, 2015).Clinical Applications of Cancer Biomarkers
The use of cancer biomarkers is having a profound impact on cancer care, especially in the era of precision medicine. Here are some of the ways biomarkers are transforming cancer care:
Personalized Therapy: Biomarkers allow treatment plans to be tailored to the individual characteristics of a patient's cancer. For example, HER2-positive breast cancer patients benefit significantly from HER2-targeted therapies, while patients with KRAS mutations can be steered away from certain treatments that are unlikely to be effective.
Monitoring Treatment Response: Biomarkers can be used to track how well a patient is responding to treatment. For example, decreasing levels of PSA in prostate cancer patients or CA-125 in ovarian cancer patients may indicate a positive response to therapy.
Predicting and Managing Resistance: Biomarkers can identify the likelihood of resistance to certain treatments. For example, patients with certain mutations in the EGFR gene may initially respond to EGFR inhibitors but develop resistance over time due to additional mutations. Regular monitoring of these biomarkers can help to adjust treatment strategies in a timely manner.
Early Detection and Screening: Biomarkers have the potential to detect cancer at an earlier, more treatable stage. For example, liquid biopsies that analyze circulating tumor DNA (ctDNA) in the blood are being developed to detect early-stage cancers and monitor for minimal residual disease.
Prognostic Information: Prognostic biomarkers provide valuable information about the likely course of the disease. For example, high levels of Ki-67, a marker of cell proliferation, may indicate more aggressive tumors and influence the intensity of treatment.
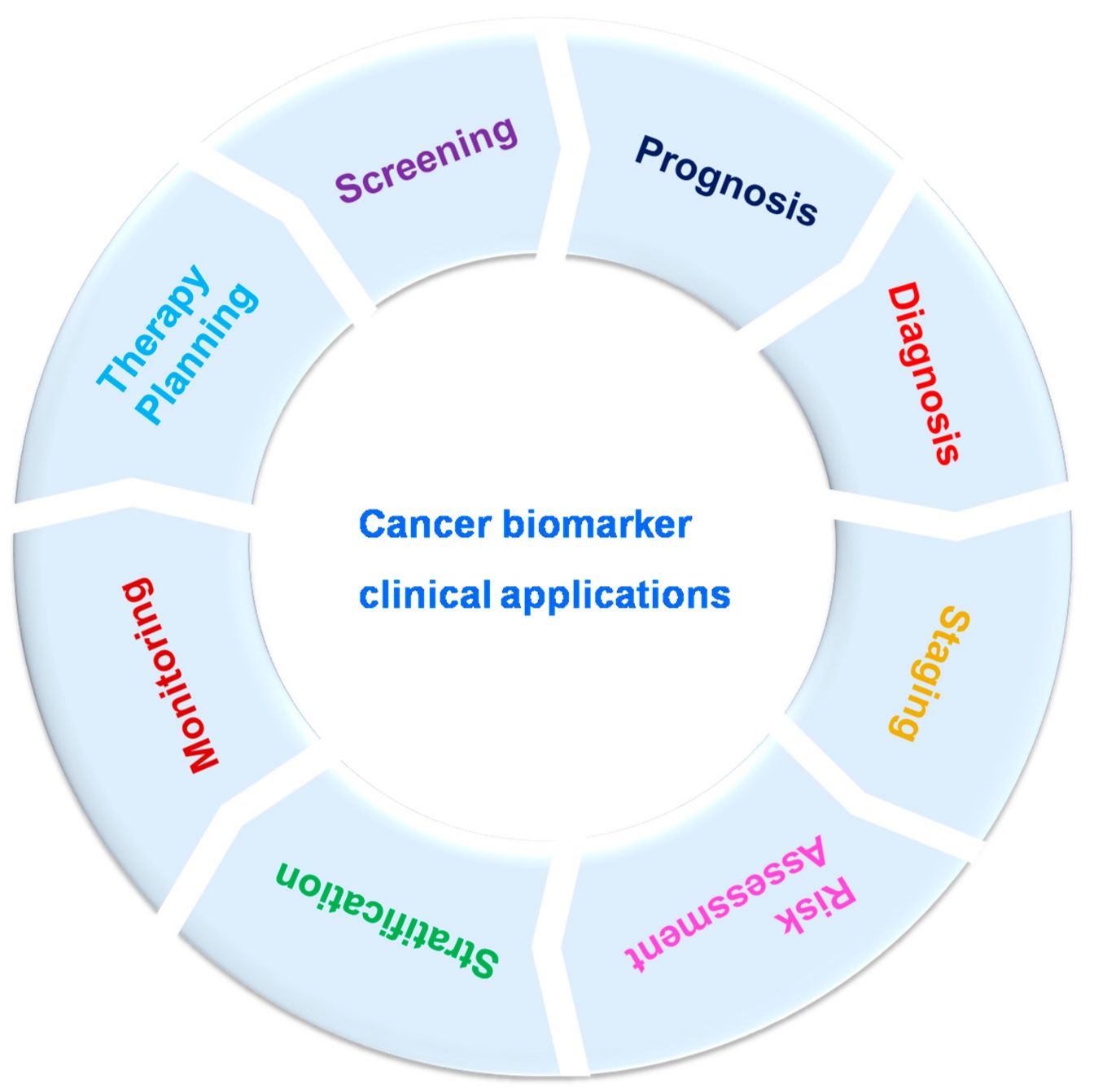 Fig. 2: Cancer biomarker clinical applications (Rao Bommi et al., 2023).
Fig. 2: Cancer biomarker clinical applications (Rao Bommi et al., 2023).Challenges and Future Directions
Despite the significant advances in cancer biomarker research, several challenges remain. One major challenge is the heterogeneity of cancer, where different parts of the same tumor or different tumors in the same patient may have distinct biomarker profiles. This complexity requires comprehensive and dynamic biomarker panels to capture the full picture.
Another challenge is the need for standardization and validation of biomarker assays to ensure consistent and reliable results across laboratories and clinical settings. Regulatory agencies such as the FDA play a critical role in this process by ensuring that biomarkers meet the necessary standards for clinical use.
Looking forward, the integration of advanced technologies such as artificial intelligence and machine learning with biomarker research holds great promise. These technologies can analyze large datasets to identify novel biomarkers and predict treatment outcomes more accurately. In addition, the development of non-invasive biomarker tests, such as liquid biopsies, will further revolutionize cancer detection and monitoring.
Case Study
Case 1: Madhavan, D.; et al. Circulating miRNAs as surrogate markers for circulating tumor cells and prognostic markers in metastatic breast cancer. Clinical Cancer Research, vol. 18, no. 21, Nov. 2012, pp. 5972–82.
The use of circulating tumor cells (CTCs) as a prognostic marker in metastatic breast cancer (MBC) is well established. However, their efficacy and accuracy are still under scrutiny, mainly due to the methods of their enrichment and identification. Circulating microRNAs (c-microRNAs, c-miRNAs) are endogenous non-coding small RNA molecules that can be secreted into the circulation, exist in remarkably stable forms, are present in almost all biological fluids, and are promising sensitive biomarkers for various diseases (oncological, cardiovascular, neurodegenerative, etc.), and their signatures accurately reflect the state of the body. Studies on the expression of microRNA markers show that they can be used to diagnose a wide range of diseases before the onset of clinical symptoms and to assess a patient's response to therapy to correct and personalize treatments. One hypothesis is that circulating miRNAs can predict the CTC status of patients with MBC.
To test this hypothesis, the researchers used the TaqMan Human MicroRNA array to analyze circulating miRNAs in the plasma of CTC-positive and CTC-negative MBC patients and healthy controls. The results show that several circulating miRNAs are elevated in CTC-positive patients compared to CTC-negative MBC patients and controls, including miR-141, miR-200a, miR-200b, miR-200c, miR-203, miR-210, miR-375, while miR-768-3p was present at lower levels in MBC cases. miR-200b emerged as the best marker to discriminate CTC-positive from CTC-negative patients. Combinations of miRNAs and miR-200b alone were found to be promising prognostic markers for progression-free and overall survival.
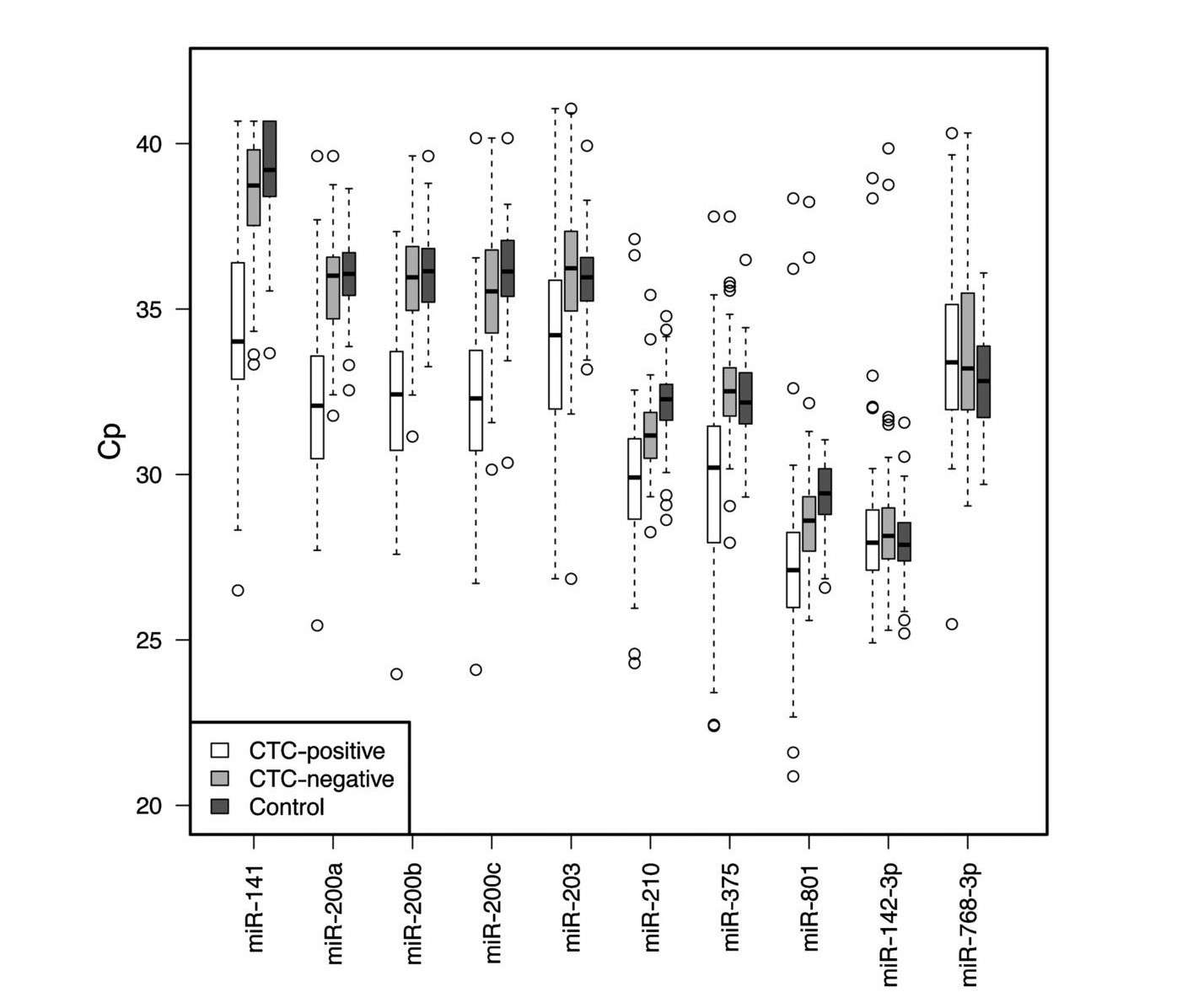 Fig. 3: Box and whisker plots of the 10 candidate miRNAs, represented as Cp values (Crossing point), across 61 CTC-positive, 72 CTC-negative MBC cases and 76 controls.
Fig. 3: Box and whisker plots of the 10 candidate miRNAs, represented as Cp values (Crossing point), across 61 CTC-positive, 72 CTC-negative MBC cases and 76 controls.Case 2: Tam, C.S.; et al. Chronic lymphocytic leukaemia CD20 expression is dependent on the genetic subtype: a study of quantitative flow cytometry and fluorescent in-situ hybridization in 510 patients. British Journal of Haematology, 2008, 141: 36-40.
CD20 is an important therapeutic target in chronic lymphocytic leukemia (CLL). To investigate the relationship between CD20 expression and cytogenetic abnormalities, we correlated the fluorescence in situ hybridization (FISH) genetic subtype of 510 treatment-naive patients with CD20 expression as measured by quantitative flow cytometry.
Patients were classified using the Dohner hierarchical classification. The median numbers of CD20 antigen sites by FISH subtypes were: 17p- (n = 26), 9341 per cell; 11q- (n = 42), 5886 per cell; +12 (n = 93), 23 603 per cell; negative FISH (n = 153), 8828 per cell; and 13q- (n = 196), 10 781 per cell. Compared to cases with negative FISH cases, 11q- cases had significantly lower CD20 expression (P = 0.001), and +12 cases had significantly higher CD20 expression (P < 0.001). Leukemic CD20 expression differed significantly between FISH subtypes. Patients with trisomy 12 CLL showed strong leukemic cell CD20 expression on leukemic cells.
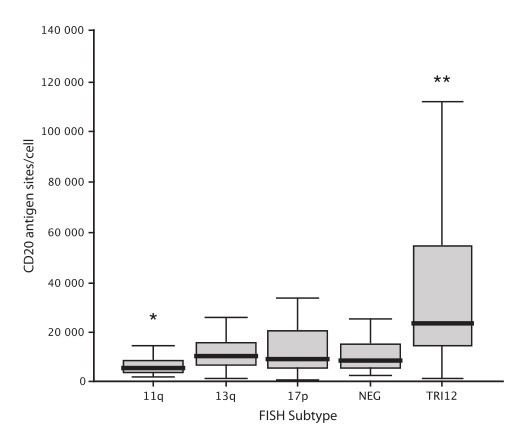 Fig. 4: Box and whisker plot of CD20 antigen expression by Dohner FISH subtype. Boundaries of the box represent the 25th and 75th percentile, and the solid line the median. *P = 0.001, **P < 0.001.
Fig. 4: Box and whisker plot of CD20 antigen expression by Dohner FISH subtype. Boundaries of the box represent the 25th and 75th percentile, and the solid line the median. *P = 0.001, **P < 0.001.Reference
- Madhavan, Dharanija, et al. "Circulating miRNAs as Surrogate Markers for Circulating Tumor Cells and Prognostic Markers in Metastatic Breast Cancer." Clinical Cancer Research, vol. 18, no. 21, Nov. 2012, pp. 5972–82. DOI.org (Crossref), https://doi.org/10.1158/1078-0432.CCR-12-1407.
- Rao Bommi, Jagadeeswara, et al. "Recent Trends in Biosensing and Diagnostic Methods for Novel Cancer Biomarkers." Biosensors, vol. 13, no. 3, Mar. 2023, p. 398. www.mdpi.com, https://doi.org/10.3390/bios13030398.
- Tam, Constantine S., et al. "Chronic Lymphocytic Leukaemia CD20 Expression Is Dependent on the Genetic Subtype: A Study of Quantitative Flow Cytometry and Fluorescent In-situ Hybridization in 510 Patients." British Journal of Haematology, vol. 141, no. 1, Apr. 2008, pp. 36–40. DOI.org (Crossref), https://doi.org/10.1111/j.1365-2141.2008.07012.x.
- Tothill, Ibtisam, and Zeynep Altintas. "Molecular Biosensors: Promising New Tools for Early Detection of Cancer." Nanobiosensors in Disease Diagnosis, Jan. 2015, p. 1. DOI.org (Crossref), https://doi.org/10.2147/NDD.S56772.
- Vaidyanathan, Kannan, and D. M. Vasudevan. "Organ Specific Tumor Markers: What's New?" Indian Journal of Clinical Biochemistry, vol. 27, no. 2, Apr. 2012, pp. 110–20. DOI.org (Crossref), https://doi.org/10.1007/s12291-011-0173-8.

