FGF Family Signaling Pathway
🧪 KRAS-2569H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-185 aa

🧪 EIF4E-2775H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-217 aa

🧪 FGF2-11H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length:
$199.00
$398
/ 100μg$699.00
$1,398
/ 1mg
🧪 AKT3-131H
Source: Insect Cells
Species: Human
Tag: GST
Conjugation:
Protein Length: 1-479 a.a.
🧪 FGFR1-138H
Source: Human Cells
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: 1-285 a.a.
🧪 FGFR2-705H
Source: HEK293
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: Met1-Glu377
🧪 FGFR4-708H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: Met1-Asp369
🧪 Fgfr4-709M
Source: HEK293
Species: Mouse
Tag: His
Conjugation:
Protein Length: Leu17-Asp366
🧪 Fgfr4-710M
Source: HEK293
Species: Mouse
Tag: Fc&His
Conjugation:
Protein Length: Met1-Asp366
🧪 FGF9-202H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: 4-208 a.a.
🧪 RAF1-1123H
Source: E.coli
Species: Human
Tag: GST
Conjugation:
Protein Length: 50-132 a.a.
🧪 EIF4EBP1-1397H
Source: E.coli
Species: Human
Tag: His
Conjugation:
Protein Length: 1-118 a.a.


🧪 STAT3-1496H
Source: Sf9 Cells
Species: Human
Tag: GST
Conjugation:
Protein Length:

🧪 STAT5A-1498H
Source: Sf9 Cells
Species: Human
Tag: His
Conjugation:
Protein Length:

🧪 PTPN11-461H
Source: E.coli
Species: Human
Tag: GST
Conjugation:
Protein Length: 246-593 a.a.
Background of FGF Family Signaling Pathway
What is FGF Family Signaling Pathway?
The signaling component of the mammalian Fibroblast Growth Factor (FGF) family is comprised of eighteen secreted proteins that interact with four signaling tyrosine kinase FGF receptors (FGFRs). FGF signaling generally follows one of three transduction pathways: Ras/MAP kinase, PI3/AKT, or PLCγ. Each pathway likely regulates specific cellular behaviors. FGFs bind to their cognate receptors and activate c-Raf-1/ ERK1 (MAPK3)/ ERK2 (MAPK1) and Rac1/ p38 MAPK cascades, both of them are involved in the regulation of cell proliferation and differentiation. Activation of PI3K/AKT(PKB) promotes cell survival and epithelial-to-mesenchymal transition. PLCγ/ PKCs cascade takes part in the regulation of development and stress response in different tissues. Inappropriate expression of FGF and improper activation of FGFRs are associated with various pathologic conditions, unregulated cell growth, and tumorigenesis.
FGF and FGFR Family
The Fibroblast Growth Factor (FGF) family is comprised of secreted signaling proteins (secreted FGFs) that signal to receptor tyrosine kinases and intracellular non-signaling proteins (intracellular FGFs (iFGFs)). Members of both branches of the FGF family are related by core sequence conservation and structure and are found in vertebrates and invertebrates. The FGF family consists of 23 peptides in mammals grouped into 6 subfamilies, including paracrine FGFs and endocrine FGFs, which exert biological effects by interacting with five different fibroblast growth factor receptors (FGFRs).
The mammalian fibroblast growth factor receptor (FGFR) family has 4 members, FGFR1, FGFR2, FGFR3, and FGFR4. The FGFRs consist of three extracellular immunoglobulin-type domains (D1-D3), a single-span trans-membrane domain and an intracellular split tyrosine kinase domain. A hallmark of FGFRs is the presence of an acidic, serine-rich sequence in the linker between D1 and D2, termed the acid box. The D2–D3 fragment of the FGFR ectodomain is necessary and sufficient for ligand binding and specificity, whereas the D1 domain and the acid box are proposed to have a role in receptor autoinhibition.
A functional FGF–FGFR unit consists of two 1:1:1 FGF–FGFR–HSGAG complexes juxtaposed in a symmetrical dimer. HSGAG facilitates FGF–FGFR dimerization by simultaneously binding both FGF and FGFR, thereby promoting and stabilizing protein-protein contacts between ligand and receptor both within the 1:1 FGF–FGFR complex and between the two complexes in the 2:2 FGF–FGFR dimer. In addition to facilitating FGF–FGFR binding, HSGAGs stabilize FGFs against degradation, act as a storage reservoir for ligand and determine the radius of ligand diffusion. Also, endocrine FGFs activate FGF receptors through members of the Klotho family.
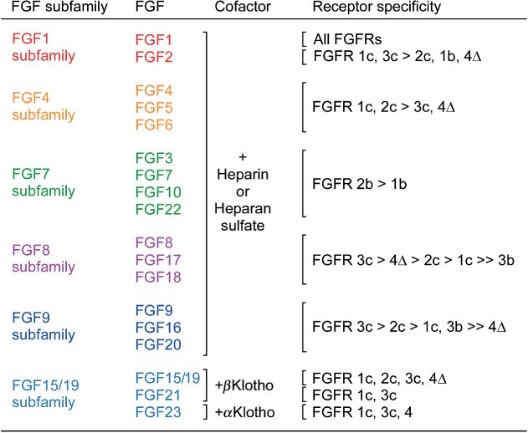
Fig1. Receptor specificity of canonical and endocrine FGFs. (David M Ornitz, 2015)
Intracellular Signal Transduction
Upon ligand binding, FGFRs form dimers, and their intracellular tyrosine kinases are activated, leading to the phosphorylation of multiple tyrosine residues. These phosphotyrosines serve as docking sites for downstream signaling molecules, initiating a cascade of events that can lead to the activation of pathways such as Ras-MAPK, PI3K-AKT, PLCγ, and STAT. These pathways ultimately affect gene expression in the nucleus.
Activation of the RAS-MAPK and PI3K-AKT pathway is initiated by phosphorylation of FRS2α. Activated (phosphorylated) FRS2α binds the membrane anchored adaptor protein, growth factor receptor-bound 2 (GRB2) and the tyrosine phosphatase SHP2. GRB2 further activates the RAS-MAPK pathway through recruitment of SOS, and the PI3K-AKT pathway through recruitment of GAB1 to the signaling complex. Phosphorylation of PLCγ by the activated FGFR tyrosine kinase leads to the hydrolysis of phosphatidylinositol 4,5-bisphosphate to produce inositol triphosphate (IP3) and diacylglycerol (DAG). The activated FGFR also phosphorylates and activates STAT1, STAT3, and STAT5, to regulate STAT pathway target gene expression.
Downstream Events of FGF Family Signaling Pathway
The downstream events of the FGF family signaling pathway are diverse and play critical roles in various cellular processes. Upon activation of the FGF receptors (FGFRs), a series of intracellular signaling cascades are initiated, leading to a wide range of biological responses. In addition to the several important signaling pathways mentioned above, here are some other key downstream events and their implications:
JNK and p38 MAPK Pathways: FGF signaling can activate the JNK (c-Jun N-terminal Kinase) and p38 MAPK (p38 Mitogen-Activated Protein Kinase) pathways, which are involved in stress responses, inflammation, and cell differentiation. These pathways are regulated by various protein kinases that are activated downstream of FGFR.
Transcriptional Regulation: The ultimate downstream effect of FGF signaling is the regulation of gene expression. Various transcription factors, including AP-1 (Activator Protein 1), CREB (cAMP Response Element-Binding Protein), and ATF2 (Activating Transcription Factor 2), are activated by the signaling pathways initiated by FGFR, leading to changes in the transcriptional profile of the cell and influencing processes such as cell growth, differentiation, and development.
Cell Cycle Progression: FGF signaling is known to influence the cell cycle, promoting cell division and proliferation. Activation of the MAPK and PI3K-AKT pathways can lead to the progression of the cell cycle, allowing cells to move from G0/G1 to the S phase, where DNA replication occurs
FGF Family Signaling Pathway Related Diseases
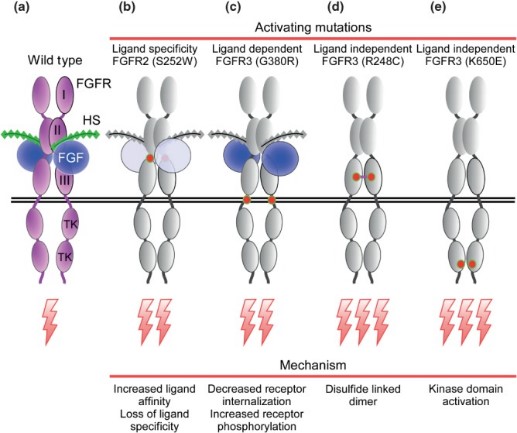
Fig2. Activating mutations in FGFRs in heritable and acquired disease. (David M Ornitz, 2015)
The FGF family signaling pathway is implicated in a variety of human diseases due to its critical roles in cellular processes such as proliferation, differentiation, migration, and metabolism. Dysregulation of this pathway can lead to developmental disorders, cancer, and metabolic diseases. Here are some of the diseases associated with the FGF family signaling pathway:
Developmental Disorders: Mutations in FGFs or FGFRs can lead to a range of developmental disorders. For example, craniosynostosis, a condition where the skull plates fuse prematurely, is associated with FGFR mutations. Other conditions such as achondroplasia and thanatophoric dysplasia are also linked to abnormalities in the FGF signaling pathway.
Cancer: Abnormal activation of the FGF/FGFR axis is observed in many types of cancer. Overexpression or amplification of FGFR genes, as well as mutations that lead to constitutive activation of the receptor, can promote uncontrolled cell growth and tumorigenesis. FGFR alterations have been identified in cancers of the breast, stomach, lung, and bladder, among others.
Metabolic Diseases: The FGF family, particularly FGF19, FGF21, and FGF23, plays a crucial role in regulating metabolism. Dysregulation of these hormones can lead to metabolic disorders such as type 2 diabetes, obesity, and non-alcoholic steatohepatitis (NASH). FGF23, for instance, is involved in the regulation of phosphate and vitamin D metabolism, and its dysfunction can lead to disorders like chronic kidney disease (CKD) and disorders of phosphate metabolism.
Craniofacial and Skeletal Disorders: FGF signaling is essential for the development of the craniofacial skeleton, and disruptions in this pathway can result in syndromes such as Apert syndrome, Crouzon syndrome, and Pfeiffer syndrome. These conditions are characterized by abnormalities in the skull and facial bones.
Neurodegenerative Diseases: FGFs have been shown to protect certain neuronal populations against neurotoxic effects in conditions like Alzheimer's disease and HIV encephalitis. Imbalances in FGF signaling may contribute to the pathogenesis of these neurodegenerative disorders.
Case Study
Case Study 1: Recombinant Human Fibroblast Growth Factor-basic (FGF2-11H)
This study sought to develop a minimally invasive intra-amniotic therapy for prenatal treatment of myelomeningocele (MMC) in an established rat model. Time-dated pregnant rats were gavage-fed retinoic acid to induce MMC. Groups received intraamniotic injections at E17.5 with alginate particles loaded with fluorescent dye, basic fibroblast growth factor (Alg-HSA-bFGF), fluorescently tagged albumin (Alginate-BSA-TR), free bFGF, blank alginate particles (Alg-Blank), or PBS. Alginate microparticles demonstrated robust binding to the MMC defect 3 h after injection. Of those specimens analyzed at E21, 150 of 239 fetuses (62.8%) were viable. Moreover, 18 of 61 (30%) treated with Alg-HSA-bFGF showed evidence of soft tissue coverage compared to 0 of 24 noninjected (P = 0.0021), 0 of 13 PBS (P = 0.0297), and 0 of 42 free bFGF (P = P < 0.0001).
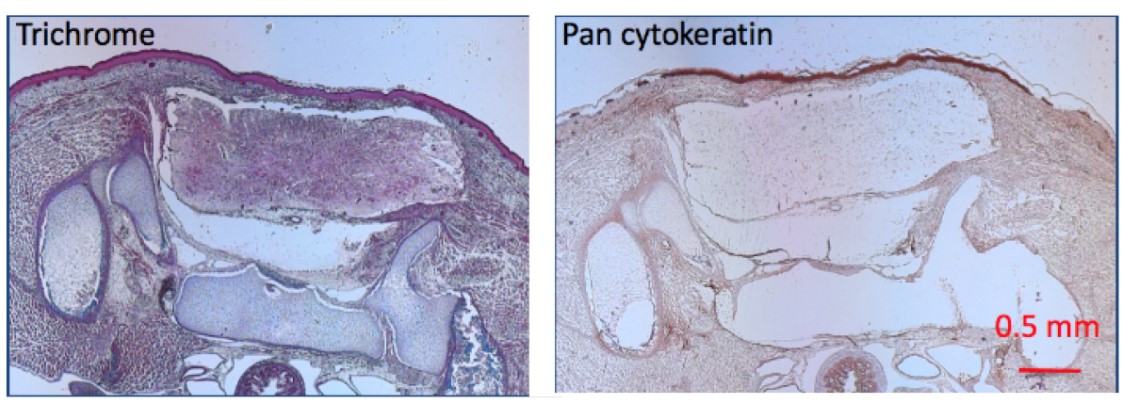
Fig1. Specimens treated with Alg-HSA-bFGF along with their associated MMC defect histology sections stained with trichrome and pan cytokeratin AE1/AE. (James S Farrelly, 2019)
Case Study 2: Recombinant Human Fibroblast Growth Factor-basic (FGF2-11H)
Injectable "smart" microspheres that are sensitive to both temperature and pH have been fabricated and tested for controlled delivery of therapeutic proteins to ischemic skeletal muscle. At 37°C and pH representative of ischemic muscle (i.e. pH 5.2-7.2), these microspheres produced sustained, diffusion-controlled release, and at normal, physiological pH (i.e. pH 7.4), they underwent dissolution and rapid clearance. Delivery of fibroblast growth factor 2 was used to confirm that protein bioactivity was retained following microsphere encapsulation/release based on a dose-dependent increase in NIH3T3 fibroblast proliferation in vitro. Microsphere-loaded or free Cy5.5-labeled albumin was injected into ischemic and control gastrocnemii of mice following unilateral induction of hind limb ischemia to model peripheral arterial disease. In the ischemic limb at days 3.5 and 7, there was higher local retention of the protein delivered via microspheres relative to injected free protein (p<0.05). However, clearance of protein delivered via microspheres was equivalent to free protein at later time points that correspond to ischemic recovery in this model. Finally, histological analysis of the gastrocnemius revealed that the polymeric microspheres did not produce any microscopic signs of toxicity near the injection site.
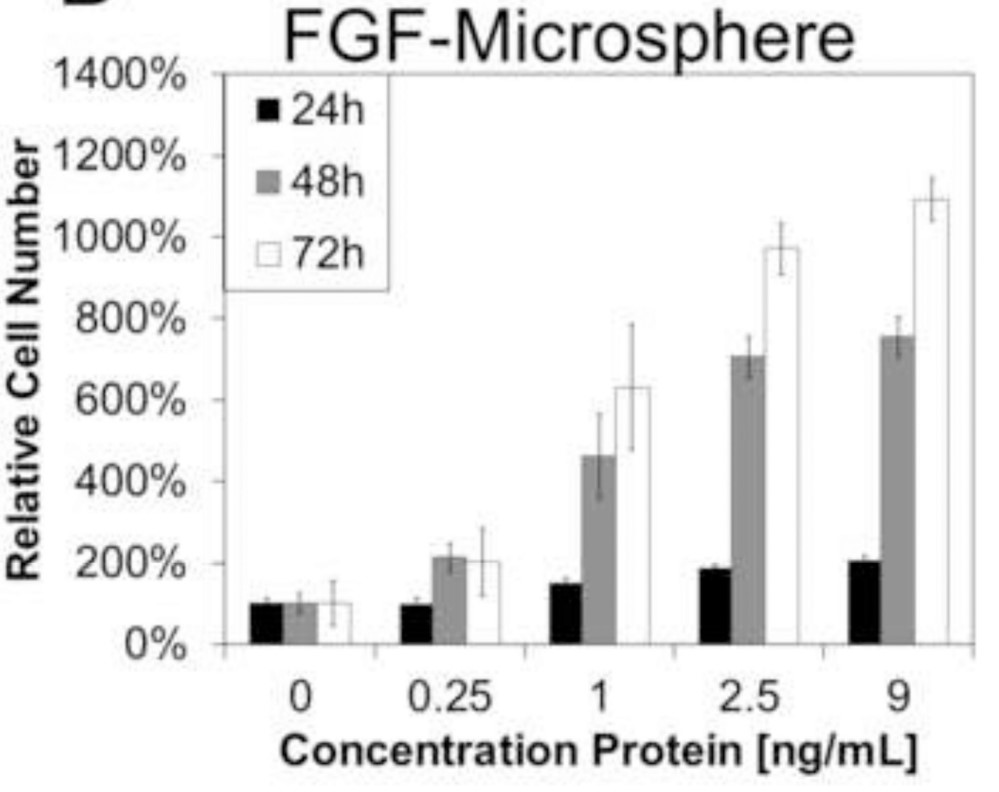
Fig2. FGF-loaded P6 microspheres mediated a time- and dose-dependent increase in the proliferation of LR3T3s. (Rucha V Joshi, 2013)
Case Study 3: Recombinant Human Fibroblast Growth Factor Receptor 1 (FGFR1-316H)
The recombinant epidermal growth factor-like domain plus the serine/threonine-rich domain of thrombomodulin (rTMD23) promotes angiogenesis and accelerates the generation of activated protein C (APC), which facilitates angiogenesis. The aim of this study was to elucidate the molecular mechanisms underlying the angiogenic activity of rTMD23. The researchers prepared rTMD23 and its mutants that did not possess the ability to promote APC generation and investigated their angiogenic activities in vitro and in vivo. Type I fibroblast growth factor receptor (FGFR1) was precipitated along with syndecan-4 by rTMD23-conjugated Sepharose in human umbilical vein endothelial cells and FGFR1-expressing human embryonic kidney 293 cells. Additionally, the kinetics of the interaction between rTMD23 and FGFR1 were analysed using surface plasmon resonance. rTMD23-induced FGFR1 activation and tube formation were inhibited by an FGFR1-specific tyrosine kinase inhibitor, PD173074, or by knockdown of FGFR1 using siRNA technology. An improvement in rat hindlimb recovery in an ischaemic model was observed following rTMD23 treatment, and this was associated with increased neovascularization and FGFR1 phosphorylation.
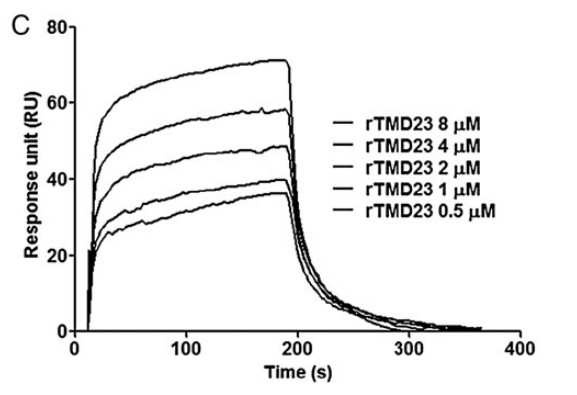
Fig3. SPR assay. The recombinant human FGFR1 was immobilized on the CM5 chip. (Cheng-Hsiang Kuo, 2015)


