Immunoglobulins superfamily
🧪 CD86-55H
Source: HEK293
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: 1-239 aa

🧪 CD4-101H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 125-202 a.a.
🧪 CD4-103H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 26-226 a.a.
🧪 CD4-104H
Source: E.coli
Species: Human
Tag: Non
Conjugation:
Protein Length: 411-459 a.a.
🧪 CD80-589H
Source: HEK293
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: 1-242 aa

🧪 CD80-590H
Source: HEK293
Species: Human
Tag: Non
Conjugation:
Protein Length: 1-242 aa


🧪 CD80-591H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: 1-242 aa

🧪 CD28-640M
Source: Human Cells
Species: Mouse
Tag: Fc&His
Conjugation:
Protein Length: 1-149 a.a.
🧪 CD48-654H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: 27-220 aa

🧪 PDGFRA-825H
Source: HEK293
Species: Human
Tag: Non
Conjugation:
Protein Length: 1-524 aa


🧪 PDGFRB-386H
Source: Sf9 Cells
Species: Human
Tag: GST
Conjugation:
Protein Length:
🧪 CD244-580H
Source: HEK293
Species: Human
Tag: Fc
Conjugation:
Protein Length: 22-221 aa

🧪 CD3D-2193H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Met1-Ala105
🧪 CD3E-2194H
Source: HEK293
Species: Human
Tag: His
Conjugation:
Protein Length: Met 1-Asp 126
🧪 CD4-2219H
Source: Human Cells
Species: Human
Tag: Fc&His
Conjugation:
Protein Length: 1-390 a.a.
SubCategories
Immunoglobulins—Product Overview
The immunoglobulin (Ig) superfamily is a large group of proteins that play essential roles in immune system function, cell adhesion, and signal transduction. Characterized by the presence of immunoglobulin-like domains, this superfamily includes both cell surface receptors and secreted molecules such as antibodies. The various members of the Ig superfamily are involved in critical processes such as regulation of the immune response, self-recognition, and defense against pathogens. Because of their importance in immunity and disease, particularly in autoimmune diseases and cancer, Ig superfamily proteins are being extensively researched for therapeutic applications. Creative BioMart offers a comprehensive range of recombinant immunoglobulin superfamily proteins and related products to support research in immunology, cell signaling and drug development.
Jump to Section
Product Families
-
Immunoglobulin-like Cell Adhesion Molecules (IgSF CAMs)
-
Antigen Receptor Accessory Molecules
-
Co-Stimulatory/Inhibitory Molecules
-
Growth Factor Receptors
-
Antigen Presenting Molecules
-
Receptor Tyrosine Kinases
-
Co-Receptors
-
Receptors on Natural Killer Cells
-
Receptors on Leukocytes
-
Cytokine Receptors
-
Ig Binding Receptors
-
Cytoskeleton
-
Other Immunoglobulins
Background
The immunoglobulin (Ig) superfamily is a diverse group of proteins that share structural similarities, most notably the presence of immunoglobulin-like domains. These domains consist of approximately 70-110 amino acids that form a β-pleated sheet structure. Members of this superfamily include antibodies, cell adhesion molecules, and receptors that are integral to immune responses, cell-cell interactions, and signal transduction. Immunoglobulin superfamily proteins are found in a wide range of species, from invertebrates to humans, reflecting their evolutionary importance.
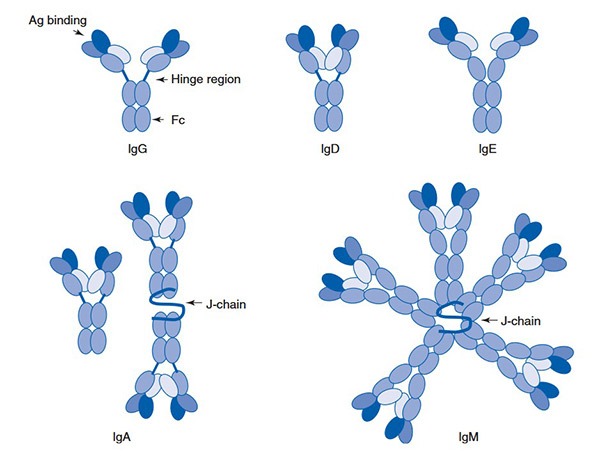
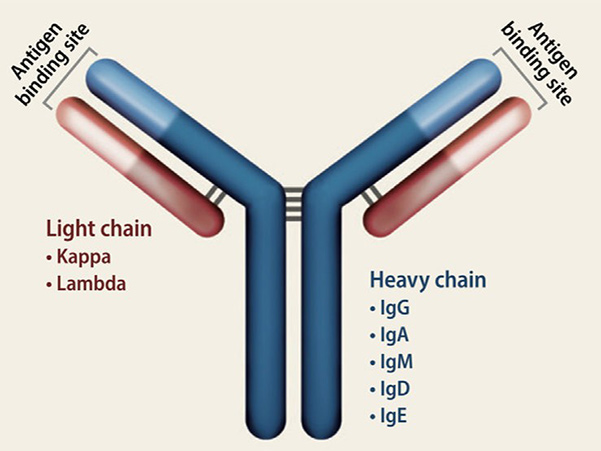
The Ig superfamily is highly diverse, consisting of over 200 proteins that vary in function, tissue distribution, and involvement in various biological processes. It includes IgG (the major class of antibodies), IgM, IgA, and IgE, as well as cell adhesion molecules such as NCAM, ICAM, and VCAM. The Ig superfamily can be divided into secreted forms, such as antibodies, and membrane-bound forms, such as receptors and adhesion molecules. This diversity supports a wide range of immune responses and cellular communication mechanisms.
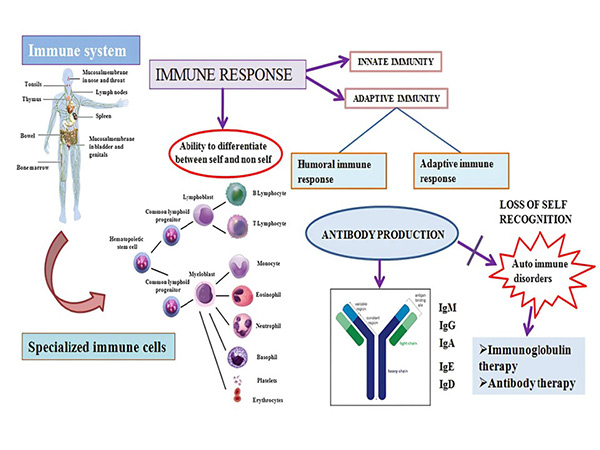
Immunoglobulin superfamily proteins are critical to the function of the immune system, facilitating cell recognition, adhesion and activation. They include antibodies (Ig), which are key to identifying and neutralizing pathogens, and receptors that mediate immune signaling, such as T cell receptors (TCRs) and B cell receptors (BCRs). In addition, Ig superfamily molecules are involved in processes such as antigen presentation, T cell activation and immune regulation. Their roles extend to cell migration, tissue development, and maintenance of cellular homeostasis.
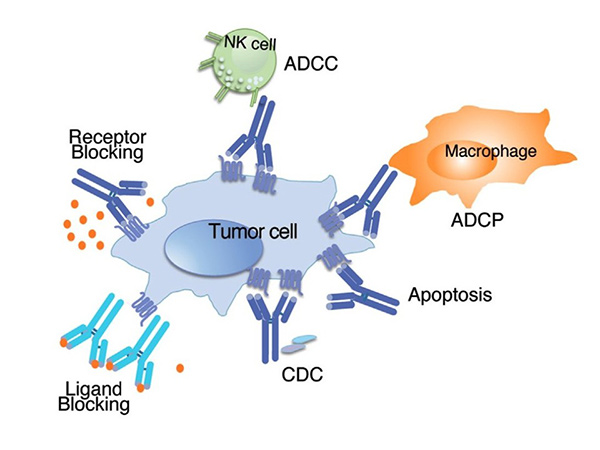
Immunoglobulin superfamily proteins have significant clinical relevance due to their involvement in immune-related diseases, including autoimmune diseases, cancer and infections. Monoclonal antibodies targeting Ig superfamily receptors, such as CTLA-4 and PD-1, have been developed for cancer immunotherapy. In addition, dysfunction of Ig proteins is associated with conditions such as immunodeficiencies and chronic inflammatory diseases. The therapeutic potential of these proteins is being actively explored in fields such as immunology, vaccine development and regenerative medicine.
Applications
Immunotherapy and Cancer Treatment
Immunoglobulin superfamily proteins have significant clinical relevance due to their involvement in immune-related diseases, including autoimmune diseases, cancer and infections. Monoclonal antibodies targeting Ig superfamily receptors, such as CTLA-4 and PD-1, have been developed for cancer immunotherapy. In addition, dysfunction of Ig proteins is associated with conditions such as immunodeficiencies and chronic inflammatory diseases. The therapeutic potential of these proteins is being actively explored in fields such as immunology, vaccine development and regenerative medicine.
Autoimmune Disease Management
Dysregulation of immunoglobulin superfamily proteins contributes to autoimmune diseases such as rheumatoid arthritis, multiple sclerosis and lupus. Therapeutic antibodies targeting Ig superfamily receptors, such as CD20 and CD4/CD28 inhibitors, help modulate the immune response and prevent excessive inflammation. These therapies are essential for restoring immune balance, reducing disease progression and improving patient outcomes in autoimmune diseases resulting from immune system overactivity.
Infectious Disease Treatment and Vaccine Development
Ig superfamily proteins, particularly antibodies (IgG, IgA, IgM), are central to immune defense against infectious diseases. They neutralize pathogens, promote immune clearance, and provide long-term immunity. Therapeutic monoclonal antibodies are used to treat infections like COVID-19, Ebola, and RSV, while vaccine research leverages Ig-mediated responses to enhance immunity. Advances in antibody engineering and passive immunization continue to improve treatment strategies for viral, bacterial, and parasitic infections.
Neurological Disorders and Regenerative Medicine
Ig superfamily proteins such as NCAM (Neural Cell Adhesion Molecule) and L1CAM are critical for neuronal growth, repair and synaptic plasticity. Their role in neural regeneration makes them promising targets for the treatment of neurodegenerative diseases such as Alzheimer's, Parkinson's and spinal cord injury. Therapies using Ig superfamily proteins aim to enhance nerve regeneration, promote neuroprotection, and restore functional connectivity in damaged neural networks, offering hope to patients with neurodegenerative and traumatic diseases.
Transplantation and Immune Tolerance
In organ transplantation, Ig superfamily proteins such as ICAM-1, VCAM-1 and CD28 influence graft rejection and immune tolerance. Targeting these proteins with immunosuppressive therapies helps to reduce the likelihood of rejection while maintaining immune protection. Monoclonal antibodies such as basiliximab (anti-IL-2Rα/CD25) are used to prevent immune-mediated damage and improve graft success rates. Research into the Ig superfamily of proteins continues to improve strategies for prolonging graft survival and minimizing transplant-related complications.
Product Features
-
High Purity & Bioactivity: Our IgSF proteins undergo rigorous purification and bioactivity validation to ensure superior quality and reliability in research applications.
-
Comprehensive Selection: We offer a diverse range of recombinant IgSF proteins, including immune checkpoint molecules (PD-1, CTLA-4), adhesion molecules (ICAM, VCAM), and cytokine receptors.
-
Endotoxin-Free & High Stability: Our proteins are produced with strict endotoxin control and optimized formulations for enhanced stability and long-term storage.
-
Multiple Expression Systems: Available in E. coli, mammalian, and insect cell systems, ensuring optimal folding, glycosylation, and functional activity.
-
Custom Protein Services: Tailored protein expression, purification, and labeling services to meet specific research and biopharmaceutical needs.
-
Bulk Supply & OEM Solutions: Scalable production for large-scale research projects, industrial applications, and contract manufacturing.
-
Application-Ready: Ideal for studies in cancer immunotherapy, autoimmune diseases, inflammation, and cell signaling research.
Case Study
Case 1: 2B4-CD48 Axis
Li et al., 2024. Suppression of adaptive NK cell expansion by macrophage-mediated phagocytosis inhibited by 2B4-CD48.
Mouse cytomegalovirus (MCMV) infection activates and expands Ly49H+ natural killer (NK) cells, which are considered adaptive or memory NK cells. This expansion is critically regulated by signaling lymphocyte activation molecule family receptors (SFRs), in particular CD48, which is highly expressed on NK cells after MCMV infection. CD48 interacts with its counter-receptor 2B4 on macrophages to inhibit the pro-phagocytic integrin LFA-1, preventing NK cell phagocytosis and promoting their expansion. This 2B4-CD48 axis plays a key role in adaptive NK cell responses with potential therapeutic implications. Adoptive transfer experiments suggest that 2B4 on recipient cells, rather than transferred NK cells, is required for Ly49 H+ NK cell expansion.
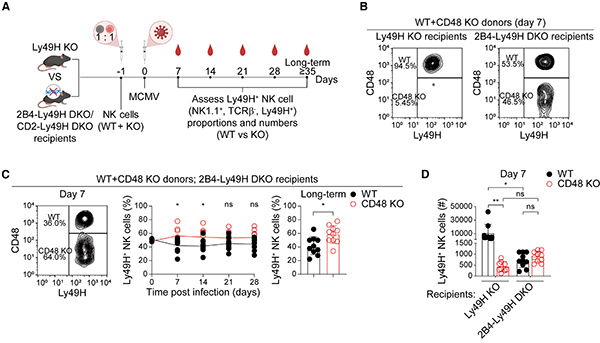
Figure 1. Recipient cell-expressed 2B4 is necessary for the expansion of Ly49H+ NK cells following MCMV infection.
Case 2: Senescence-Driven PD-L2 Immunoevasion
Chaib et al., 2024. The efficacy of chemotherapy is limited by intratumoral senescent cells expressing PD-L2.
Chemotherapy induces senescent cancer cells that modify the tumor microenvironment, promoting immunosuppression and tumor growth. A proteomics screen revealed that PD-L2, an immune checkpoint inhibitor, is highly upregulated in senescent cancer cells, enabling them to evade immune clearance. PD-L2-deficient tumors fail to recruit myeloid-derived suppressor cells and undergo regression via CD8 T cells post-chemotherapy. PD-L2 knockout (KO) tumors are highly susceptible to chemotherapy, with significantly reduced growth and prolonged survival in mice. Combining chemotherapy with anti-PD-L2 blockade enhances tumor remission, offering a promising therapeutic strategy.
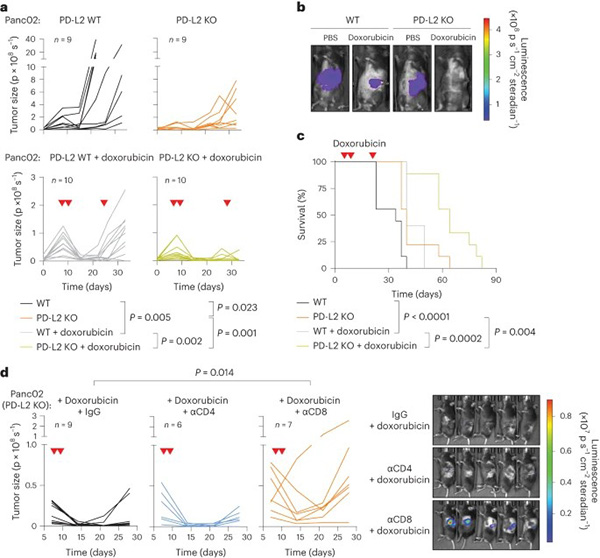
Figure 2. A combination of PD-L2 ablation and chemotherapy results in CD8 T cell-dependent tumor remission.
FAQs
-
Q: What are Immunoglobulin Superfamily proteins?
A: Immunoglobulin superfamily (IgSF) proteins are a diverse group of cell surface and soluble proteins that contain immunoglobulin-like domains. They play crucial roles in immune response, cell adhesion, and signal transduction, making them essential in immunology, cancer research, and therapeutic development. -
Q: What types of Immunoglobulin Superfamily products do you offer?
A: We provide a wide range of recombinant IgSF proteins, monoclonal and polyclonal antibodies, fusion proteins, and assay kits. Our catalog includes key immune checkpoint molecules (PD-1, CTLA-4), adhesion molecules (ICAM, VCAM), and cytokine receptors for research and therapeutic applications.
-
Q: How can Immunoglobulin Superfamily proteins be used in research?
A: IgSF proteins are widely used in cancer immunotherapy studies, autoimmune disease modeling, vaccine development, and cell signaling research. They are essential tools for investigating immune interactions, T-cell activation, and therapeutic antibody development.
-
Q: How do you ensure the quality of your Immunoglobulin Superfamily products?
A: Our products undergo rigorous quality control, including purity analysis, bioactivity testing, and endotoxin level assessment. We ensure high reproducibility and batch-to-batch consistency for reliable research results. -
Q: Do you offer customized Immunoglobulin Superfamily proteins?
A: Yes, we provide custom production services for IgSF proteins, including protein expression, purification, and labeling. We also offer tailored antibody development to meet specific research needs. -
Q: What is the difference between immunoglobulin and antibody?
A: Immunoglobulin (Ig) is a general term for a group of glycoproteins that function as antibodies or immune receptors. Antibodies are a specific type of immunoglobulin produced by B cells in response to antigens, helping to neutralize pathogens and mediate immune responses. -
Q: Are immunoglobulin therapies the same as antibody therapies?
A: Not necessarily. Immunoglobulin therapies (e.g., IVIG) involve pooled antibodies from donors to boost immunity in immunodeficient patients. Monoclonal antibody therapies, on the other hand, use lab-engineered antibodies targeting specific diseases like cancer and autoimmune disorders.

